Catecholamine exocytosis during low frequency stimulation in mouse adrenal chromaffin cells is primarily asynchronous and controlled by the novel mechanism of Ca2+ syntilla suppression
- PMID: 25128575
- PMCID: PMC4253468
- DOI: 10.1113/jphysiol.2014.278127
Catecholamine exocytosis during low frequency stimulation in mouse adrenal chromaffin cells is primarily asynchronous and controlled by the novel mechanism of Ca2+ syntilla suppression
Abstract
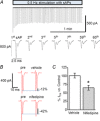
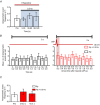
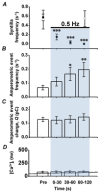
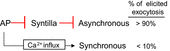
Adrenal chromaffin cells (ACCs), stimulated by the splanchnic nerve, generate action potentials (APs) at a frequency near 0.5 Hz in the resting physiological state, at times described as 'rest and digest'. How such low frequency stimulation in turn elicits sufficient catecholamine exocytosis to set basal sympathetic tone is not readily explained by the classical mechanism of stimulus-secretion coupling, where exocytosis is synchronized to AP-induced Ca(2+) influx. By using simulated action potentials (sAPs) at 0.5 Hz in isolated patch-clamped mouse ACCs, we show here that less than 10% of all catecholaminergic exocytosis, measured by carbon fibre amperometry, is synchronized to an AP. The asynchronous phase, the dominant phase, of exocytosis does not require Ca(2+) influx. Furthermore, increased asynchronous exocytosis is accompanied by an AP-dependent decrease in frequency of Ca(2+) syntillas (i.e. transient, focal Ca(2+) release from internal stores) and is ryanodine sensitive. We propose a mechanism of disinhibition, wherein APs suppress Ca(2+) syntillas, which themselves inhibit exocytosis as they do in the case of spontaneous catecholaminergic exocytosis.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures





References
-
- Becker PL. Fay FS. Photobleaching of fura-2 and its effect on determination of calcium concentrations. Am J Physiol. 1987;253:C613–618. - PubMed
-
- Cannell MB, Cheng H. Lederer WJ. The control of calcium release in heart muscle. Science. 1995;268:1045–1049. - PubMed
-
- Cheng H, Lederer WJ. Cannell MB. Calcium sparks: elementary events underlying excitation–contraction coupling in heart muscle. Science. 1993;262:740–744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

