A genetic mechanism for Tibetan high-altitude adaptation
- PMID: 25129147
- PMCID: PMC4473257
- DOI: 10.1038/ng.3067
A genetic mechanism for Tibetan high-altitude adaptation
Abstract
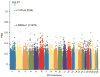
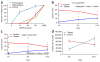
Tibetans do not exhibit increased hemoglobin concentration at high altitude. We describe a high-frequency missense mutation in the EGLN1 gene, which encodes prolyl hydroxylase 2 (PHD2), that contributes to this adaptive response. We show that a variant in EGLN1, c.[12C>G; 380G>C], contributes functionally to the Tibetan high-altitude phenotype. PHD2 triggers the degradation of hypoxia-inducible factors (HIFs), which mediate many physiological responses to hypoxia, including erythropoiesis. The PHD2 p.[Asp4Glu; Cys127Ser] variant exhibits a lower K(m) value for oxygen, suggesting that it promotes increased HIF degradation under hypoxic conditions. Whereas hypoxia stimulates the proliferation of wild-type erythroid progenitors, the proliferation of progenitors with the c.[12C>G; 380G>C] mutation in EGLN1 is significantly impaired under hypoxic culture conditions. We show that the c.[12C>G; 380G>C] mutation originated ∼8,000 years ago on the same haplotype previously associated with adaptation to high altitude. The c.[12C>G; 380G>C] mutation abrogates hypoxia-induced and HIF-mediated augmentation of erythropoiesis, which provides a molecular mechanism for the observed protection of Tibetans from polycythemia at high altitude.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Prchal JT. Production of Erythrocytes. McGraw Hill; New York: 2010.
-
- Prchal JT. Secondary Polycythemia (Erythrocytosis) McGraw Hill; New York: 2010. pp. 823–839.
-
- Simonson TS, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329:72–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HG005846/HG/NHGRI NIH HHS/United States
- P01CA108671/CA/NCI NIH HHS/United States
- T32 HL098062/HL/NHLBI NIH HHS/United States
- P01 CA108671/CA/NCI NIH HHS/United States
- I01 BX001140/BX/BLRD VA/United States
- R00 HG005846/HG/NHGRI NIH HHS/United States
- K99 HG005846/HG/NHGRI NIH HHS/United States
- K08 HL119355/HL/NHLBI NIH HHS/United States
- R00 HL118215/HL/NHLBI NIH HHS/United States
- HL119355-01/HL/NHLBI NIH HHS/United States
- UL1 TR001067/TR/NCATS NIH HHS/United States
- HL098062/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

