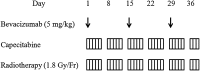Neoadjuvant capecitabine, bevacizumab and radiotherapy for locally advanced rectal cancer: results of a single-institute Phase I study
- PMID: 25129557
- PMCID: PMC4229928
- DOI: 10.1093/jrr/rru063
Neoadjuvant capecitabine, bevacizumab and radiotherapy for locally advanced rectal cancer: results of a single-institute Phase I study
Abstract
The aim of this Phase I clinical trial was to assess the feasibility and safety of capecitabine-based preoperative chemoradiotherapy (CRT) combined with bevacizumab and to determine the optimal capecitabine dose for Japanese patients with locally advanced rectal cancer. Patients with cT3/T4 rectal cancer were eligible. Bevacizumab was administered at 5 mg/kg intravenously on Days 1, 15 and 29. Capecitabine was administered on weekdays concurrently with pelvic radiotherapy at a daily dose of 1.8 Gy, totally to 50.4 Gy. Capecitabine was initiated at 825 mg/m(2) twice daily at Dose Level 1, with a planned escalation to 900 mg/m(2) twice daily at Dose Level 2. Within 6.1-10.3 (median, 9.4) weeks after the completion of the CRT, surgery was performed. Three patients were enrolled at each dose level. Regarding the CRT-related acute toxicities, all of the adverse events were limited to Grade 1. There was no Grade 2 or greater toxicity. No patient needed attenuation or interruption of bevacizumab, capecitabine or radiation. All of the patients received the scheduled dose of CRT. All of the patients underwent R0 resection. Two (33.3%) of the six patients had a pathological complete response, and five (83.3%) patients experienced downstaging. In total, three patients (50%) developed postoperative complications. One patient developed an intrapelvic abscess and healed with incisional drainage. The other two patients healed following conservative treatment. This regimen was safely performed as preoperative CRT for Japanese patients with locally advanced rectal cancer. The recommended capecitabine dose is 900 mg/m(2) twice daily.
Keywords: bevacizumab; capecitabine; chemoradiotherapy; rectal cancer.
© The Author 2014. Published by Oxford University Press on behalf of The Japan Radiation Research Society and Japanese Society for Radiation Oncology.
Similar articles
-
Phase 2 study of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5-fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: ECOG 3204.Cancer. 2013 Apr 15;119(8):1521-7. doi: 10.1002/cncr.27890. Epub 2013 Jan 3. Cancer. 2013. PMID: 23288663 Free PMC article. Clinical Trial.
-
Phase II trial of preoperative radiochemotherapy with concurrent bevacizumab, capecitabine and oxaliplatin in patients with locally advanced rectal cancer.Radiat Oncol. 2013 Apr 15;8:90. doi: 10.1186/1748-717X-8-90. Radiat Oncol. 2013. PMID: 23587311 Free PMC article. Clinical Trial.
-
Preoperative radiation therapy with concurrent capecitabine, bevacizumab, and erlotinib for rectal cancer: a phase 1 trial.Int J Radiat Oncol Biol Phys. 2014 Feb 1;88(2):301-5. doi: 10.1016/j.ijrobp.2013.10.034. Epub 2013 Dec 5. Int J Radiat Oncol Biol Phys. 2014. PMID: 24315563 Free PMC article. Clinical Trial.
-
Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer--a two stage phase II clinical trial.Radiother Oncol. 2012 Jan;102(1):10-3. doi: 10.1016/j.radonc.2011.06.008. Epub 2011 Jul 7. Radiother Oncol. 2012. PMID: 21741716 Clinical Trial.
-
Combined vascular endothelial growth factor-targeted therapy and radiotherapy for rectal cancer: theory and clinical practice.Semin Oncol. 2006 Oct;33(5 Suppl 10):S35-40. doi: 10.1053/j.seminoncol.2006.08.007. Semin Oncol. 2006. PMID: 17145523 Free PMC article. Review.
Cited by
-
A phase II trial of preoperative concurrent chemotherapy and dose escalated intensity modulated radiotherapy (IMRT) for locally advanced rectal cancer.J Cancer. 2017 Sep 6;8(16):3114-3121. doi: 10.7150/jca.21237. eCollection 2017. J Cancer. 2017. PMID: 29158782 Free PMC article.
References
-
- Kapiteijin E, Maijnen CAM, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46. - PubMed
-
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomised trials. Lancet. 2001;358:1291–304. - PubMed
-
- Bosset JF, Collette L, Callais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–23. - PubMed
-
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–7. - PubMed
-
- Kusters M, Beets GL, van de Velde CJH, et al. A comparison between the treatment of low rectal cancer in Japan and the Netherlands, focusing on the patterns of local recurrence. Annals of Surg. 2009;249:229–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials