Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function
- PMID: 25129603
- PMCID: PMC4155940
- DOI: 10.1016/j.jtcvs.2014.06.038
Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function
Abstract
Objectives: Cell-based angiogenic therapy for ischemic heart failure has had limited clinical impact, likely related to low cell retention (<1%) and dispersion. We developed a novel, tissue-engineered, hydrogel-based cell-delivery strategy to overcome these limitations and provide prolonged regional retention of myocardial endothelial progenitor cells at high cell dosage.
Methods: Endothelial progenitor cells were isolated from Wistar rats and encapsulated in fibrin gels. In vitro viability was quantified using a fluorescent live-dead stain of transgenic enhanced green fluorescent protein(+) endothelial progenitor cells. Endothelial progenitor cell-laden constructs were implanted onto ischemic rat myocardium in a model of acute myocardial infarction (left anterior descending ligation) for 4 weeks. Intramyocardial cell injection (2 × 10(6) endothelial progenitor cells), empty fibrin, and isolated left anterior descending ligation groups served as controls. Hemodynamics were quantified using echocardiography, Doppler flow analysis, and intraventricular pressure-volume analysis. Vasculogenesis and ventricular geometry were quantified. Endothelial progenitor cell migration was analyzed by using endothelial progenitor cells from transgenic enhanced green fluorescent protein(+) rodents.
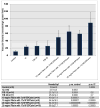
Results: Endothelial progenitor cells demonstrated an overall 88.7% viability for all matrix and cell conditions investigated after 48 hours. Histologic assessment of 1-week implants demonstrated significant migration of transgenic enhanced green fluorescent protein(+) endothelial progenitor cells from the fibrin matrix to the infarcted myocardium compared with intramyocardial cell injection (28 ± 12.3 cells/high power field vs 2.4 ± 2.1 cells/high power field, P = .0001). We also observed a marked increase in vasculogenesis at the implant site. Significant improvements in ventricular hemodynamics and geometry were present after endothelial progenitor cell-hydrogel therapy compared with control.
Conclusions: We present a tissue-engineered, hydrogel-based endothelial progenitor cell-mediated therapy to enhance cell delivery, cell retention, vasculogenesis, and preservation of myocardial structure and function.
Copyright © 2014 The American Association for Thoracic Surgery. Published by Mosby, Inc. All rights reserved.
Figures


References
-
- Araszkiewicz A, Grajek S, Lesiak M, et al. Effect of impaired myocardial reperfusion on left ventricular remodeling in patients with anterior wall acute myocardial infarction treated with primary coronary intervention. The American journal of cardiology. 2006;98:725–8. - PubMed
-
- Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–6. - PubMed
-
- Hofmann M, Wollert KC, Meyer GP, et al. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

