Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis
- PMID: 25130259
- PMCID: PMC4143920
- DOI: 10.1038/ncomms5632
Myc inhibition is effective against glioma and reveals a role for Myc in proficient mitosis
Abstract
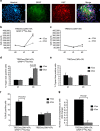
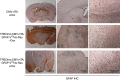
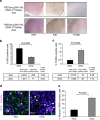
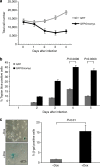
Gliomas are the most common primary tumours affecting the adult central nervous system and respond poorly to standard therapy. Myc is causally implicated in most human tumours and the majority of glioblastomas have elevated Myc levels. Using the Myc dominant negative Omomyc, we previously showed that Myc inhibition is a promising strategy for cancer therapy. Here, we preclinically validate Myc inhibition as a therapeutic strategy in mouse and human glioma, using a mouse model of spontaneous multifocal invasive astrocytoma and its derived neuroprogenitors, human glioblastoma cell lines, and patient-derived tumours both in vitro and in orthotopic xenografts. Across all these experimental models we find that Myc inhibition reduces proliferation, increases apoptosis and remarkably, elicits the formation of multinucleated cells that then arrest or die by mitotic catastrophe, revealing a new role for Myc in the proficient division of glioma cells.
Figures


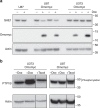
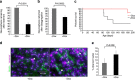
References
-
- Kleihues P., Burger P. C. & Scheithauer B. W. The new WHO classification of brain tumours. Brain Pathol. 3, 255–268 (1993). - PubMed
-
- Wen P. Y. & Kesari S. Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507 (2008). - PubMed
-
- Huse J. T. & Holland E. C. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 10, 319–331 (2010). - PubMed
-
- Guha A., Feldkamp M. M., Lau N., Boss G. & Pawson A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene 15, 2755–2765 (1997). - PubMed
-
- Feldkamp M. M., Lau N., Rak J., Kerbel R. S. & Guha A. Normoxic and hypoxic regulation of vascular endothelial growth factor (VEGF) by astrocytoma cells is mediated by Ras. Int. J. Cancer 81, 118–124 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

