Microglial regulation of immunological and neuroprotective functions of astroglia
- PMID: 25130274
- PMCID: PMC4237670
- DOI: 10.1002/glia.22738
Microglial regulation of immunological and neuroprotective functions of astroglia
Abstract
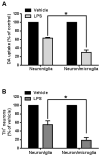
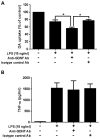
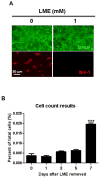
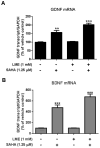
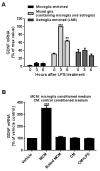
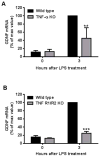
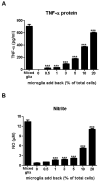
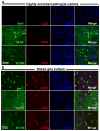
Microglia and astroglia play critical roles in the development, function, and survival of neurons in the CNS. However, under inflammatory conditions the role of astrogliosis in the inflammatory process and its effects on neurons remains unclear. Here, we used several types of cell cultures treated with the bacterial inflammogen LPS to address these questions. We found that the presence of astroglia reduced inflammation-driven neurotoxicity, suggesting that astrogliosis is principally neuroprotective. Neutralization of supernatant glial cell line-derived neurotrophic factor (GDNF) released from astroglia significantly reduced this neuroprotective effect during inflammation. To determine the immunological role of astroglia, we optimized a highly-enriched astroglial culture protocol and demonstrated that LPS failed to induce the synthesis and release of TNF-α and iNOS/NO. Instead we found significant enhancement of TNF-α and iNOS expression in highly-enriched astroglial cultures required the presence of 0.5-1% microglia, respectively. Thus suggesting that microglial-astroglial interactions are required for LPS to induce the expression of pro-inflammatory factors and GDNF from astroglia. Specifically, we found that microglia-derived TNF-α plays a pivotal role as a paracrine signal to regulate the neuroprotective functions of astrogliosis. Taken together, these findings suggest that astroglia may not possess the ability to directly recognize the innate immune stimuli LPS, but rather depend on crosstalk with microglia to elicit release of neurotrophic factors as a counterbalance to support neuronal survival from the collateral damage generated by activated microglia during neuroinflammation.
Keywords: astroglia; glial cell line-derived neurotrophic factor; glial interaction; microglia; neuroinflammation; neuroprotection.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures


References
-
- Bell MD, Lopez-Gonzalez R, Lawson L, Hughes D, Fraser I, Gordon S, Perry VH. Upregulation of the macrophage scavenger receptor in response to different forms of injury in the CNS. Journal of neurocytology. 1994;23:605–13. - PubMed
-
- Beutner C, Linnartz-Gerlach B, Schmidt SV, Beyer M, Mallmann MR, Staratschek-Jox A, Schultze JL, Neumann H. Unique transcriptome signature of mouse microglia. Glia. 2013;61:1429–42. - PubMed
-
- Biber K, Owens T, Boddeke E. What is microglia neurotoxicity (Not)? Glia. 2014;62:841–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

