Targeting of viral capsids to nuclear pores in a cell-free reconstitution system
- PMID: 25131140
- PMCID: PMC5524173
- DOI: 10.1111/tra.12209
Targeting of viral capsids to nuclear pores in a cell-free reconstitution system
Abstract
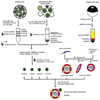
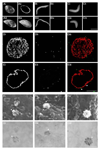
Many viruses deliver their genomes into the nucleoplasm for viral transcription and replication. Here, we describe a novel cell-free system to elucidate specific interactions between viruses and nuclear pore complexes (NPCs). Nuclei reconstituted in vitro from egg extracts of Xenopus laevis, an established biochemical system to decipher nuclear functions, were incubated with GFP-tagged capsids of herpes simplex virus, an alphaherpesvirus replicating in the nucleus. Capsid binding to NPCs was analyzed using fluorescence and field emission scanning electron microscopy. Tegument-free capsids or viral capsids exposing inner tegument proteins on their surface bound to nuclei, while capsids inactivated by a high-salt treatment or covered by inner and outer tegument showed less binding. There was little binding of the four different capsid types to nuclei lacking functional NPCs. This novel approach provides a powerful system to elucidate the molecular mechanisms that enable viral structures to engage with NPCs. Furthermore, this assay could be expanded to identify molecular cues triggering viral genome uncoating and nuclear import of viral genomes.
Keywords: Xenopus nuclei; herpes simplex virus; herpesviruses; nuclear pore; reconstitution.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures


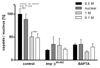
References
-
- Frenkiel-Krispin D, Maco B, Aebi U, Medalia O. Structural analysis of a metazoan nuclear pore complex reveals a fused concentric ring architecture. J Mol Biol. 2010;395:578–586. - PubMed
-
- Maimon T, Elad N, Dahan I, Medalia O. The human nuclear pore complex as revealed by cryo-electron tomography. Structure. 2012;20:998–1006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

