Understanding the determinants of substrate specificity in IMP family metallo-β-lactamases: the importance of residue 262
- PMID: 25131397
- PMCID: PMC4287004
- DOI: 10.1002/pro.2530
Understanding the determinants of substrate specificity in IMP family metallo-β-lactamases: the importance of residue 262
Abstract
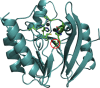
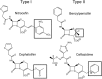
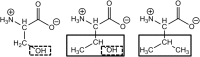
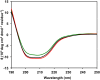
In Gram-negative bacteria, resistance to β-lactam antibacterials is largely due to β-lactamases and is a growing public health threat. One of the most concerning β-lactamases to evolve in bacteria are the Class B enzymes, the metallo-β-lactamases (MBLs). To date, penams and cephems resistant to hydrolysis by MBLs have not yet been found. As a result of this broad substrate specificity, a better understanding of the role of catalytically important amino acids in MBLs is necessary to design novel β-lactams and inhibitors. Two MBLs, the wild type IMP-1 with serine at position 262, and an engineered variant with valine at the same position (IMP-1-S262V), were previously found to exhibit very different substrate spectra. These findings compelled us to investigate the impact of a threonine at position 262 (IMP-1-S262T) on the substrate spectrum. Here, we explore MBL sequence-structure-activity relationships by predicting and experimentally validating the effect of the S262T substitution in IMP-1. Using site-directed mutagenesis, threonine was introduced at position 262, and the IMP-1-S262T enzyme, as well as the other two enzymes IMP-1 and IMP-1-S262V, were purified and kinetic constants were determined against a range of β-lactam antibacterials. Catalytic efficiencies (kcat /KM ) obtained with IMP-1-S262T and minimum inhibitory concentrations (MICs) observed with bacterial cells expressing the protein were intermediate or comparable to the corresponding values with IMP-1 and IMP-1-S262V, validating the role of this residue in catalysis. Our results reveal the important role of IMP residue 262 in β-lactam turnover and support this approach to predict activities of certain novel MBL variants.
Keywords: IMP-1 antibody; antibiotic resistance; enzyme evolution; metallo-β-lactamase; point mutation.
© 2014 The Protein Society.
Figures

References
-
- Ambler RP. The structure of β-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289:321–331. - PubMed
-
- Crowder MW, Spencer J, Vila AJ. Metallo-β-lactamases: novel weaponry for antibiotic resistance in bacteria. Acc Chem Res. 2006;39:721–728. - PubMed
-
- Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem Pharmacol. 2007;74:1686–1701. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

