HDL and cognition in neurodegenerative disorders
- PMID: 25131449
- PMCID: PMC4252583
- DOI: 10.1016/j.nbd.2014.07.015
HDL and cognition in neurodegenerative disorders
Abstract
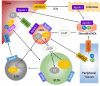
High-density lipoproteins (HDLs) are a heterogeneous group of lipoproteins composed of various lipids and proteins. HDL is formed both in the systemic circulation and in the brain. In addition to being a crucial player in the reverse cholesterol transport pathway, HDL possesses a wide range of other functions including anti-oxidation, anti-inflammation, pro-endothelial function, anti-thrombosis, and modulation of immune function. It has been firmly established that high plasma levels of HDL protect against cardiovascular disease. Accumulating evidence indicates that the beneficial role of HDL extends to many other systems including the central nervous system. Cognition is a complex brain function that includes all aspects of perception, thought, and memory. Cognitive function often declines during aging and this decline manifests as cognitive impairment/dementia in age-related and progressive neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease, Huntington's disease, and amyotrophic lateral sclerosis. A growing concern is that no effective therapy is currently available to prevent or treat these devastating diseases. Emerging evidence suggests that HDL may play a pivotal role in preserving cognitive function under normal and pathological conditions. This review attempts to summarize recent genetic, clinical and experimental evidence for the impact of HDL on cognition in aging and in neurodegenerative disorders as well as the potential of HDL-enhancing approaches to improve cognitive function.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Akhmedova SN, et al. Paraoxonase 1 Met--Leu 54 polymorphism is associated with Parkinson's disease. J Neurol Sci. 2001;184:179–82. - PubMed
-
- Akhondzadeh S, et al. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomized and placebo-controlled trial. J Clin Pharm Ther. 2003;28:53–9. - PubMed
-
- Albers JJ, et al. Cholesteryl ester transfer protein in human brain. Int J Clin Lab Res. 1992;21:264–6. - PubMed
-
- Alzheimer's Association 2013 Alzheimer's disease facts and figures. Alzheimers Dement. 2013;9:208–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

