4-bromopropofol decreases action potential generation in spinal neurons by inducing a glycine receptor-mediated tonic conductance
- PMID: 25131750
- PMCID: PMC4290717
- DOI: 10.1111/bph.12880
4-bromopropofol decreases action potential generation in spinal neurons by inducing a glycine receptor-mediated tonic conductance
Abstract
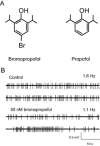
Background and purpose: Impaired function of spinal strychnine-sensitive glycine receptors gives rise to chronic pain states and movement disorders. Therefore, increased activity of glycine receptors should help to treat such disorders. Although compounds targeting glycine receptors with a high selectivity are lacking, halogenated analogues of propofol have recently been considered as potential candidates. Therefore we asked whether 4-bromopropofol attenuated the excitability of spinal neurons by promoting glycine receptor-dependent inhibition.
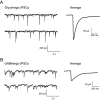
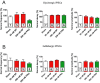
Experimental approach: The actions of sub-anaesthetic concentrations of propofol and 4-bromopropofol were investigated in spinal tissue cultures prepared from mice. Drug-induced alterations in action potential firing were monitored by extracellular multi-unit recordings. The effects on GABAA and glycine receptor-mediated inhibition were quantified by whole-cell voltage-clamp recordings.
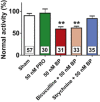
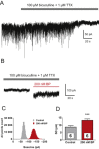
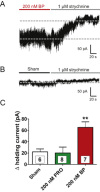
Key results: Low concentrations of 4-bromopropofol (50 nM) reduced action potential activity of ventral horn neurons by about 30%, compared with sham-treated slices. This effect was completely abolished by strychnine (1 μM). In voltage-clamped neurons, 4-bromopropofol activated glycine receptors, generating a tonic current of 65 ± 10 pA, while GABAA - and glycine receptor-mediated synaptic transmission remained unaffected.
Conclusions and implications: The highest glycine levels in the CNS are found in the ventral horn of the spinal cord, a region mediating pain-induced motor reflexes and participating in the control of muscle tone. 4-Bromopropofol may serve as a starting point for the development of non-sedative, non-addictive, muscle relaxants and analgesics to be used to treat low back pain.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
ALX 1393 inhibits spontaneous network activity by inducing glycinergic tonic currents in the spinal ventral horn.Neuroscience. 2013 Dec 3;253:165-71. doi: 10.1016/j.neuroscience.2013.08.042. Epub 2013 Aug 29. Neuroscience. 2013. PMID: 23994185
-
Propofol and sevoflurane depress spinal neurons in vitro via different molecular targets.Anesthesiology. 2004 Nov;101(5):1167-76. doi: 10.1097/00000542-200411000-00017. Anesthesiology. 2004. PMID: 15505453
-
The actions of propofol on gamma-aminobutyric acid-A and glycine receptors in acutely dissociated spinal dorsal horn neurons of the rat.Anesth Analg. 2002 Oct;95(4):907-14, table of contents. doi: 10.1097/00000539-200210000-00021. Anesth Analg. 2002. PMID: 12351266
-
Propofol modulates phasic and tonic GABAergic currents in spinal ventral horn interneurones.Br J Anaesth. 2015 Mar;114(3):491-8. doi: 10.1093/bja/aeu269. Epub 2014 Aug 23. Br J Anaesth. 2015. PMID: 25150989
-
Effects of isoflurane and enflurane on GABAA and glycine receptors contribute equally to depressant actions on spinal ventral horn neurones in rats.Br J Anaesth. 2006 Nov;97(5):687-94. doi: 10.1093/bja/ael239. Epub 2006 Sep 13. Br J Anaesth. 2006. PMID: 16973644
Cited by
-
Causality of genetically determined serum metabolites on lower back pain or/and sciatica: a comprehensive Mendelian randomized study.Front Pain Res (Lausanne). 2024 Sep 25;5:1370704. doi: 10.3389/fpain.2024.1370704. eCollection 2024. Front Pain Res (Lausanne). 2024. PMID: 39385756 Free PMC article.
-
Activation and modulation of recombinant glycine and GABAA receptors by 4-halogenated analogues of propofol.Br J Pharmacol. 2016 Nov;173(21):3110-3120. doi: 10.1111/bph.13566. Epub 2016 Sep 6. Br J Pharmacol. 2016. PMID: 27459129 Free PMC article.
-
Glycine receptors and glycine transporters: targets for novel analgesics?Cell Mol Life Sci. 2018 Feb;75(3):447-465. doi: 10.1007/s00018-017-2622-x. Epub 2017 Aug 8. Cell Mol Life Sci. 2018. PMID: 28791431 Free PMC article. Review.
-
Phosphorylation state-dependent modulation of spinal glycine receptors alleviates inflammatory pain.J Clin Invest. 2016 Jul 1;126(7):2547-60. doi: 10.1172/JCI83817. Epub 2016 Jun 6. J Clin Invest. 2016. PMID: 27270175 Free PMC article.
References
-
- Ahrens J, Leuwer M, Stachura S, Krampfl K, Belelli D, Lambert J, et al. A transmembrane residue influences the interaction of propofol with the strychnine-sensitive glycine alpha1 and alpha1 beta receptor. Anesth Analg. 2008;107:1875–1883. - PubMed
-
- Antkowiak B, Heck D. Effects of the volatile anesthetic enflurane on spontaneous discharge rate and GABA(A)-mediated inhibition of Purkinje cells in rat cerebellar slices. J Neurophysiol. 1997;77:2525–2538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

