Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review
- PMID: 25131812
- PMCID: PMC6936726
- DOI: 10.1016/j.cct.2014.08.004
Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review
Abstract
Background: Underrepresentation of racial and ethnic minorities in clinical trials remains a reality while they have disproportionately higher rates of health disparities.
Objective: The purpose of this study was to identify successful community-engaged interventions that included health care providers as a key strategy in addressing barriers to clinical trial enrollment of underrepresented patients.
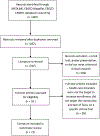
Design: A systematic review of the literature on interventions addressing enrollment barriers to clinical trials for racial and ethnic minorities was performed in Ovid MEDLINE, EBSCO Megafile, and EBSCO CINAHL. The systematic review identified 360 studies, and 20 were selected using the inclusion criteria. An iterative process extracted information from the eligible studies.
Results: The 20 selected studies were analyzed and then grouped by first author, nature of the clinical research initiative, priority populations, key strategies, and study outcomes. Nine of the studies addressed cancer clinical trials and 11 related to chronic medical conditions, including diabetes, hypertension management, and chronic kidney disease. The key strategies employed were categorized according to their presumed impact on barriers incurred at distinct steps in study recruitment: clinical trial awareness, opportunity to participate, and acceptance of enrollment. The strategies were further categorized by whether they would address barriers associated with minority perceptions of the research process and barriers related to how studies were designed and implemented.
Conclusion: Multiple and flexible strategies targeting providers and participants at provider sites and within communities might be needed to enroll underrepresented populations into clinical trials.
Keywords: Clinical trials; Minority; Recruitment.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures
References
-
- National Institutes of Health. Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research – Amended. In: Health NIo, editor. Washington, D.C.: Department of Health and Human Services; 2001.
-
- Spiker CA, Weinberg AD. Policies to address disparities in clinical trials: the EDICT Project. Journal of cancer education : the official journal of the American Association for Cancer Education. 2009;24 Suppl 2:S39–49. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical