Exposure to rufinamide and risks of CNS adverse events in drug-resistant epilepsy: a meta-analysis of randomized, placebo-controlled trials
- PMID: 25132372
- PMCID: PMC4256616
- DOI: 10.1111/bcp.12479
Exposure to rufinamide and risks of CNS adverse events in drug-resistant epilepsy: a meta-analysis of randomized, placebo-controlled trials
Abstract
Aim: Epilepsy is a complex disease necessitating continuous development of new therapeutic strategies to encounter drug-resistant cases. Among new adjuvant antiepileptic drugs, rufinamide is structurally distinct from other antiepileptic drugs. It is used to treat partial-onset seizures and seizures associated with Lennox-Gastaut syndrome (LGS) in adult and children. To date, there has been no attempt to evaluate systematically the risks of adverse events with rufinamide.
Methods: We performed a quantitative risk analysis of central nervous system (CNS) adverse events of rufinamide from all randomized, double-blind, add-on, placebo-controlled trials. The meta-analysis was undertaken with fixed effects models.
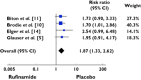
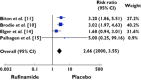
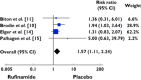
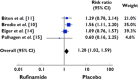
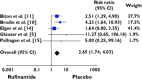
Results: Of the 886 publications reviewed, 99 papers were retrieved and five articles met the inclusion criteria. One thousand two hundred and fifty-two patients were included. Our study showed that exposure to rufinamide was associated with a significant increase in risk of somnolence [relative ratio (RR) 1.87; 95% confidence interval (CI) 1.33, 2.62; P = 0.0003], dizziness (RR 2.66; 95% CI 2.00, 3.55; P = 0.00001), fatigue (RR 2.14; 95% CI 1.57, 2.91; P = 0.01) and headache (RR 1.28; 95% CI 1.02, 1.59, P = 0.03). In addition, exposure to rufinamide was associated with higher treatment discontinuation rates as compared with placebo (RR 2.65; 95% CI 1.74, 4.03; P = 0.00001).
Conclusions: The risk of CNS adverse events appears to be increased in patients exposed to rufinamide as well as the treatment discontinuation rates. However, although statistical associations were significant, additional long term safety studies are required to confirm the clinical significance of these findings, as most reports described only mild and moderate adverse events.
Keywords: adults; central nervous system; children; epilepsy; rufinamide; safety.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
Rufinamide add-on therapy for drug-resistant epilepsy.Cochrane Database Syst Rev. 2020 Nov 8;11(11):CD011772. doi: 10.1002/14651858.CD011772.pub3. Cochrane Database Syst Rev. 2020. PMID: 33179247 Free PMC article.
-
Rufinamide: a new antiepileptic medication for the treatment of seizures associated with lennox-gastaut syndrome.Ann Pharmacother. 2010 Apr;44(4):658-67. doi: 10.1345/aph.1M679. Epub 2010 Mar 16. Ann Pharmacother. 2010. PMID: 20233912 Review.
-
The efficacy and safety of rufinamide in drug-resistant epilepsy: A meta-analysis of double-blind, randomized, placebo controlled trials.Epilepsy Res. 2016 Feb;120:104-10. doi: 10.1016/j.eplepsyres.2016.01.001. Epub 2016 Jan 7. Epilepsy Res. 2016. PMID: 26811934
-
Safety and tolerability of rufinamide in children with epilepsy: a pooled analysis of 7 clinical studies.J Child Neurol. 2009 Dec;24(12):1520-5. doi: 10.1177/0883073809350508. J Child Neurol. 2009. PMID: 19955344
-
Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome.Neurology. 2008 May 20;70(21):1950-8. doi: 10.1212/01.wnl.0000303813.95800.0d. Epub 2008 Apr 9. Neurology. 2008. PMID: 18401024 Clinical Trial.
Cited by
-
The Pharmacology and Toxicology of Third-Generation Anticonvulsant Drugs.J Med Toxicol. 2017 Dec;13(4):329-342. doi: 10.1007/s13181-017-0626-4. Epub 2017 Aug 16. J Med Toxicol. 2017. PMID: 28815428 Free PMC article. Review.
-
Antiepileptic Drug Treatment in Children with Epilepsy.CNS Drugs. 2015;29(10):847-63. doi: 10.1007/s40263-015-0281-8. CNS Drugs. 2015. PMID: 26400189 Free PMC article. Review.
-
Pharmacokinetics and Drug Interaction of Antiepileptic Drugs in Children and Adolescents.Paediatr Drugs. 2018 Oct;20(5):429-453. doi: 10.1007/s40272-018-0302-4. Paediatr Drugs. 2018. PMID: 30003498 Review.
References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. - PubMed
-
- French JA. Refractory epilepsy: clinical overview. Epilepsia. 2007;48:3–7. - PubMed
-
- Joseph JR, Schultz RJ, Wilfong AA. Rufinamide for refractory epilepsy in a pediatric and young adult population. Epilepsy Res. 2011;93:87–89. - PubMed
-
- Glauser T, Kluger G, Sachdeo R, Krauss G, Perdomo C, Arroyo S. Rufinamide for generalized seizures associated with Lennox-Gastaut syndrome. Neurology. 2008;70:1950–1958. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

