Efficacy and tolerability of treatment with azacitidine for 5 days in elderly patients with acute myeloid leukemia
- PMID: 25132519
- PMCID: PMC4298384
- DOI: 10.1002/cam4.321
Efficacy and tolerability of treatment with azacitidine for 5 days in elderly patients with acute myeloid leukemia
Abstract
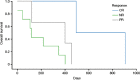
Acute myeloid leukemia (AML) patients aged ≥ 60 years tolerate standard induction chemotherapy poorly. Therapy with azacitidine at a dose of 75 mg/m(2)/day for 7 days appears to be better tolerated, and is approved by the Food and Drug Administration (FDA) for the treatment of elderly AML patients with bone marrow (BM) blast counts of 20-30%. Here, we report the results of a prospective, phase 2, open-label study that evaluated the tolerability and efficacy of a 5-day regimen of single-agent subcutaneous azacitidine 100 mg/m(2)/day administered every 28 days in 15 elderly patients with newly diagnosed AML, 14 of whom had BM blast counts >30%. The overall response rate was 47%. Complete remission, partial remission, and hematologic improvement were achieved by 20, 13, and 13% of patients, respectively. Median overall survival was 355 days for the entire cohort, and 532 days for responders. Median time to best response was 95 days, and median treatment duration was 198 days (range = 13-724 days). Grade 3-4 hematologic toxicities comprised predominantly febrile neutropenia (40%) and thrombocytopenia (20%). Febrile neutropenia was the most common cause of hospitalization. Nonhematologic toxicities, consisting of injection-site skin reactions and fatigue (Grades 1-2), occurred in 73% (n = 11) of patients. No treatment-related deaths occurred during the study. The dose and schedule of therapy remained constant in all but four patients. The findings of this study suggest that administration of subcutaneous azacitidine 100 mg/m(2)/day for 5 days every 28 days is a feasible, well-tolerated, and effective alternative to standard induction chemotherapy in elderly patients with AML.
Keywords: Azacitidine; acute myeloid leukemia; aged; outpatients; subcutaneous injections.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures
References
-
- American Cancer Society. 2014. Cancer Facts and Figures 2013. American Cancer Society, 201, Atlanta, GA. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume... (accessed 15 February 2014)
-
- Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. - PubMed
-
- Büchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Muller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J. Clin. Oncol. 2009;27:61–69. - PubMed
-
- Löwenberg B, Zittoun R, Kerkhofs H, Jehn U, Abels J, Debusscher L, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65 + years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J. Clin. Oncol. 1989;7:1268–1274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials