Mitochondrial and cellular mechanisms for managing lipid excess
- PMID: 25132820
- PMCID: PMC4116787
- DOI: 10.3389/fphys.2014.00282
Mitochondrial and cellular mechanisms for managing lipid excess
Abstract
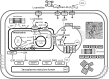
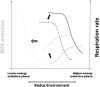
Current scientific debates center on the impact of lipids and mitochondrial function on diverse aspects of human health, nutrition and disease, among them the association of lipotoxicity with the onset of insulin resistance in skeletal muscle, and with heart dysfunction in obesity and diabetes. Mitochondria play a fundamental role in aging and in prevalent acute or chronic diseases. Lipids are main mitochondrial fuels however these molecules can also behave as uncouplers and inhibitors of oxidative phosphorylation. Knowledge about the functional composition of these contradictory effects and their impact on mitochondrial-cellular energetics/redox status is incomplete. Cells store fatty acids (FAs) as triacylglycerol and package them into cytoplasmic lipid droplets (LDs). New emerging data shows the LD as a highly dynamic storage pool of FAs that can be used for energy reserve. Lipid excess packaging into LDs can be seen as an adaptive response to fulfilling energy supply without hindering mitochondrial or cellular redox status and keeping low concentration of lipotoxic intermediates. Herein we review the mechanisms of action and utilization of lipids by mitochondria reported in liver, heart and skeletal muscle under relevant physiological situations, e.g., exercise. We report on perilipins, a family of proteins that associate with LDs in response to loading of cells with lipids. Evidence showing that in addition to physical contact, mitochondria and LDs exhibit metabolic interactions is presented and discussed. A hypothetical model of channeled lipid utilization by mitochondria is proposed. Direct delivery and channeled processing of lipids in mitochondria could represent a reliable and efficient way to maintain reactive oxygen species (ROS) within levels compatible with signaling while ensuring robust and reliable energy supply.
Keywords: beta-oxidation; energetics; lipid droplet; palmitoyl CoA; perilipin; reactive oxygen species; redox environment.
Figures
References
-
- Anderson E. J., Kypson A. P., Rodriguez E., Anderson C. A., Lehr E. J., Neufer P. D. (2009b). Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J. Am. Coll. Cardiol. 54, 1891–1898 10.1016/j.jacc.2009.07.031 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

