In vitro effects of polyphenols on colorectal cancer cells
- PMID: 25132926
- PMCID: PMC4133796
- DOI: 10.4251/wjgo.v6.i8.289
In vitro effects of polyphenols on colorectal cancer cells
Abstract
Aim: To investigate the effects of quercetin and genistein on colon cancer cell proliferation and their estrogen receptor β (ERβ) expression.
Methods: Colon cancer cells were stably transfected with a mammalian expression vector to overexpress ERβ (HCT8-β8-expressing cells) or a control vector (HCT8-pSV2neo-expressing cells). The proliferation of these cells was examined after treatment with quercetin or genistein (5-100 μmol/L), or 10 nmol/L 17β-estradiol (17β-E2). Cell viability was examined by acridine orange staining following treatments for 48 or 144 h. Effects of quercetin and genistein on ERβ transcriptional transactivation were examined by luciferase activity in HCT8-β8-expressing cells transiently transfected with a pEREtkLUC reporter vector. In addition, the regulation of ERβ transcription by phytoestrogens and 17β-E2 was examined by quantitative polymerase chain reaction.
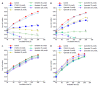
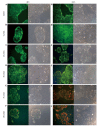
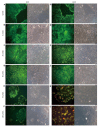
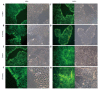
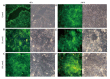
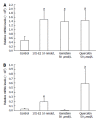
Results: Proliferation of HCT8-β8-expressing cells was not reduced low doses (5 μmol/L) of quercetin and genistein, while it was reduced at 25-50 μmol/L with an effect similar to 10 nmol/L 17β-E2. Treatment with doses of phytoestrogens ≥ 75 μmol/L completely blocked cell growth and reduced overall cell counts, however no effects at any dose were observed in HCT8-pSV2neo-expressing cells. These results were supported by viability staining that revealed acridine orange-stained lysosomes with high doses or extended treatment periods. Genistein and quercetin (50 μmol/L) significantly increased ER-responsive luciferase activity similar to 10 nmol/L 17β-E2 (P < 0.05). Furthermore, genistein and quercetin (50 μmol/L), as well as 10 nmol/L 17β-E2 significantly increased ERβ mRNA levels in HCT8-β8-expressing cells (P < 0.05). In addition, treatment of HCT8-pSV2neo-expressing cells with 50 µmol/L quercetin or 10 nmol/L 17β-E2 significantly increased ERβ mRNA levels compared to untreated controls (P < 0.05), though the absolute levels were much lower than in HCT8-β8-expressing cells.
Conclusion: The antitumorigenic effects of the phytoestrogenic compounds quercetin and genistein on colon cancers cells occur through ERβ activity and expression.
Keywords: Estrogen receptor; Genistein; HCT8-pSV2neo; HCT8-β8 cells; Quercetin.
Figures

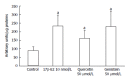
References
-
- American Cancer Society. Cancer Facts and Figures 2010. Atlanta: American Cancer Society; 2010. Available from: http: //www.cancer.org/acs/groups/content/@nho/documents/document/acspc-....
-
- Chen L, Crawford JM. Tratto Gastrointestinale. In: Robbins and Cotran., editor. Pathologic Basis of Disease, 7th ed. Milan: Elsevier; 2006. pp. 797–877.
-
- Lacassagne A. Endocrine factors concerned in the genesis of experimental mammary carcinoma. J Endocrinol. 1955;13:ix–xviii. - PubMed
-
- American Cancer Society. Cancer Facts and Figures 2007. Atlanta: American Cancer Society; 2007. Available from: http: //www.cancer.org/acs/groups/content/@nho/documents/document/caff20....
LinkOut - more resources
Full Text Sources
Other Literature Sources

