Efficacy and day 7 plasma piperaquine concentrations in African children treated for uncomplicated malaria with dihydroartemisinin-piperaquine
- PMID: 25133389
- PMCID: PMC4136730
- DOI: 10.1371/journal.pone.0103200
Efficacy and day 7 plasma piperaquine concentrations in African children treated for uncomplicated malaria with dihydroartemisinin-piperaquine
Abstract
Background: One promising new Artemisinin-based combination therapies (ACTs) is dihydroartemisinin-piperaquine (DHA-PQ). However, the pharmacokinetics of piperaquine and the relationship between drug levels and clinical efficacy are incompletely characterized, particularly in children.
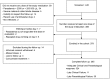
Methods: We performed a single-arm open-label trial in Bobo-Dioulasso, Burkina Faso. A total of 379 participants aged 6 months or more with uncomplicated falciparum malaria were enrolled. Each participant received daily dose of DHA-PQ for three days and followed for 42 days. Parasitological efficacy was analyzed, considering rates of recrudescence and overall recurrence. PK was an exploratory endpoint and a priori, no sample size had been determined. Day 7 capillary and venous plasma concentrations of piperaquine were measured in children aged 2-10 years.
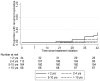
Results: Of the 379 participants, 365 (96.3%) completed 42 days of follow-up. The median daily dose of PQ was 18.5 mg/kg [6.5-24]. Treatment with DHA-PQ was well tolerated with fever and parasitemia resolution within 48 hours in nearly all children. Recurrent malaria within 42 days of follow-up occurred in 31.3% (10/34) of children less than 2 years old, 16.0% (16/106) of those aged 2-5 years, 9.4% (15/160) of those aged 5-10 years, and none (0/68) of those over 10 years old. After genotyping, 3 of 41 recurrent episodes were recrudescence. An exploratory analysis shows that children with successful treatment outcomes had significantly higher median plasma concentrations of PQ compared to those with recurrent malaria within 42 days after therapy, considering either capillary samples (68 ng/ml [50-85] compared to 48 ng/ml [36-55], p<0.001) or venous samples (42 ng/ml [29-59] compared to 25 ng/ml [19-44], p<0.001).
Conclusion: DHA-PQ was effective for uncomplicated P. falciparum malaria treatment and offers an alternative to other ACTs. Recurrent malaria was mainly due to new infections after treatment and was correlated with low day 7 PQ concentration in the youngest patients.
Trial registration: Controlled-Trials.com ISRCTN59761234.
Conflict of interest statement
Figures

References
-
- WHO (2010) Guidelines for the treatment of malaria, second edition.
-
- Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, et al. (2005) Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 365: 1474–1480. - PubMed
-
- Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, et al. (2007) Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet 369: 491–498. - PubMed
-
- Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, et al. (2007) Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. Jama 297: 2210–2219. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

