PAX7 expression defines germline stem cells in the adult testis
- PMID: 25133429
- PMCID: PMC4153705
- DOI: 10.1172/JCI75943
PAX7 expression defines germline stem cells in the adult testis
Abstract
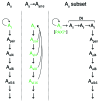
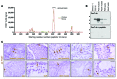
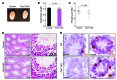
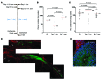
Spermatogenesis is a complex, multistep process that maintains male fertility and is sustained by rare germline stem cells. Spermatogenic progression begins with spermatogonia, populations of which express distinct markers. The identity of the spermatogonial stem cell population in the undisturbed testis is controversial due to a lack of reliable and specific markers. Here we identified the transcription factor PAX7 as a specific marker of a rare subpopulation of A(single) spermatogonia in mice. PAX7+ cells were present in the testis at birth. Compared with the adult testis, PAX7+ cells constituted a much higher percentage of neonatal germ cells. Lineage tracing in healthy adult mice revealed that PAX7+ spermatogonia self-maintained and produced expanding clones that gave rise to mature spermatozoa. Interestingly, in mice subjected to chemotherapy and radiotherapy, both of which damage the vast majority of germ cells and can result in sterility, PAX7+ spermatogonia selectively survived, and their subsequent expansion contributed to the recovery of spermatogenesis. Finally, PAX7+ spermatogonia were present in the testes of a diverse set of mammals. Our data indicate that the PAX7+ subset of A(single) spermatogonia functions as robust testis stem cells that maintain fertility in normal spermatogenesis in healthy mice and mediate recovery after severe germline injury, such as occurs after cancer therapy.
Figures



Comment in
-
The quest for male germline stem cell markers: PAX7 gets ID'd.J Clin Invest. 2014 Oct;124(10):4219-22. doi: 10.1172/JCI77926. Epub 2014 Aug 26. J Clin Invest. 2014. PMID: 25157826 Free PMC article.
References
-
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21(6):776–798. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

