A selective microRNA-based strategy inhibits restenosis while preserving endothelial function
- PMID: 25133430
- PMCID: PMC4153706
- DOI: 10.1172/JCI76069
A selective microRNA-based strategy inhibits restenosis while preserving endothelial function
Abstract
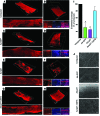
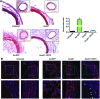
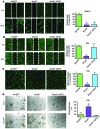
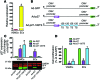
Drugs currently approved to coat stents used in percutaneous coronary interventions do not discriminate between proliferating vascular smooth muscle cells (VSMCs) and endothelial cells (ECs). This lack of discrimination delays reendothelialization and vascular healing, increasing the risk of late thrombosis following angioplasty. We developed a microRNA-based (miRNA-based) approach to inhibit proliferative VSMCs, thus preventing restenosis, while selectively promoting reendothelialization and preserving EC function. We used an adenoviral (Ad) vector that encodes cyclin-dependent kinase inhibitor p27(Kip1) (p27) with target sequences for EC-specific miR-126-3p at the 3' end (Ad-p27-126TS). Exogenous p27 overexpression was evaluated in vitro and in a rat arterial balloon injury model following transduction with Ad-p27-126TS, Ad-p27 (without miR-126 target sequences), or Ad-GFP (control). In vitro, Ad-p27-126TS protected the ability of ECs to proliferate, migrate, and form networks. At 2 and 4 weeks after injury, Ad-p27-126TS-treated animals exhibited reduced restenosis, complete reendothelialization, reduced hypercoagulability, and restoration of the vasodilatory response to acetylcholine to levels comparable to those in uninjured vessels. By incorporating miR-126-3p target sequences to leverage endogenous EC-specific miR-126, we overexpressed exogenous p27 in VSMCs, while selectively inhibiting p27 overexpression in ECs. Our proof-of-principle study demonstrates the potential of using a miRNA-based strategy as a therapeutic approach to specifically inhibit vascular restenosis while preserving EC function.
Figures


Comment in
-
Healing the injured vessel wall using microRNA-facilitated gene delivery.J Clin Invest. 2014 Sep;124(9):3694-7. doi: 10.1172/JCI77509. Epub 2014 Aug 18. J Clin Invest. 2014. PMID: 25133421 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

