Redox and metabolic regulation of stem/progenitor cells and their niche
- PMID: 25133592
- PMCID: PMC4175426
- DOI: 10.1089/ars.2014.5931
Redox and metabolic regulation of stem/progenitor cells and their niche
Abstract
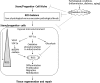
Stem cells are defined as cells that have the capacity to self-renew and exhibit multipotency or pluripotency, whereas progenitor cells are committed to selected lineages but retain their self-renewal capacity. The stem or progenitor cell niche refers to the microenvironment of the regenerative cells in the bone marrow (BM) or other tissues such as the heart. It can regulate self-renewal, differentiation, migration, and proliferation of regenerative stem/progenitor cells. The precise regulatory mechanisms by which the niche and the stem/progenitor cells interact are an active area of research. Reactive oxygen species (ROS) are one such niche regulatory mechanism. Quiescent stem cells in a hypoxic niche exhibit low ROS levels due to well-organized antioxidant defense systems, which protect stem cells from extrinsic oxidative stress, whereas high levels of ROS promote the differentiation or migration of stem/progenitor cells. In pathophysiological conditions such as diabetes, BM niche dysfunction induced by oxidative stress contributes to the reduction of the angiogenic and vasculogenic potential of BM-derived regenerative cells, thereby leading to less efficient healing and revascularization. Cells have evolved mechanisms to fine-tune ROS levels by tightly regulated metabolic pathways such as glycolysis rather than oxidative phosphorylation to reduce oxidative stress. This Forum will summarize the recent progress regarding the redox and metabolic regulation of hematopoietic and cardiac stem/progenitor cells, as well as their niche interactions involved in tissue regeneration and repair under physiological and pathological conditions. Understanding such mechanisms will contribute to the development of novel therapeutic strategies to enhance regeneration and repair of diseased tissues.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

