Curation and analysis of multitargeting agents for polypharmacological modeling
- PMID: 25133604
- PMCID: PMC4170814
- DOI: 10.1021/ci500092j
Curation and analysis of multitargeting agents for polypharmacological modeling
Abstract
In drug discovery and development, the conventional "single drug, single target" concept has been shifted to "single drug, multiple targets"--a concept coined as polypharmacology. For studies in this emerging field, dedicated and high-quality databases of multitargeting ligands would be exceedingly beneficial. To this end, we conducted a comprehensive analysis of the structural and chemical/biological profiles of polypharmacological agents and present a Web-based database (Polypharma). All of these compounds curated herein have been cocrystallized with more than one unique protein with intensive reports of their multitargeting activities. The present study provides more insight of drug multitargeting and is particularly useful for polypharmacology modeling. This specialized curation has been made publically available at http:/imdlab.org/polypharma/
Figures

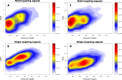
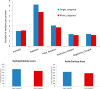

Similar articles
-
Polypharmacology by Design: A Medicinal Chemist's Perspective on Multitargeting Compounds.J Med Chem. 2019 Jan 24;62(2):420-444. doi: 10.1021/acs.jmedchem.8b00760. Epub 2018 Aug 3. J Med Chem. 2019. PMID: 30035545
-
Accessing Expert-Curated Pharmacological Data in the IUPHAR/BPS Guide to PHARMACOLOGY.Curr Protoc Bioinformatics. 2018 Mar;61(1):1.34.1-1.34.46. doi: 10.1002/cpbi.46. Curr Protoc Bioinformatics. 2018. PMID: 30040201
-
The IUPHAR/BPS Guide to PHARMACOLOGY: an expert-driven knowledgebase of drug targets and their ligands.Nucleic Acids Res. 2014 Jan;42(Database issue):D1098-106. doi: 10.1093/nar/gkt1143. Epub 2013 Nov 14. Nucleic Acids Res. 2014. PMID: 24234439 Free PMC article.
-
Modeling Polypharmacological Profiles by Affinity Fingerprinting.Curr Pharm Des. 2016;22(46):6885-6894. doi: 10.2174/1381612822666160831104718. Curr Pharm Des. 2016. PMID: 27587199 Review.
-
Multitargeting Compounds: A Promising Strategy to Overcome Multi-Drug Resistant Tuberculosis.Molecules. 2020 Mar 9;25(5):1239. doi: 10.3390/molecules25051239. Molecules. 2020. PMID: 32182964 Free PMC article. Review.
Cited by
-
An up-to-date overview of computational polypharmacology in modern drug discovery.Expert Opin Drug Discov. 2020 Sep;15(9):1025-1044. doi: 10.1080/17460441.2020.1767063. Epub 2020 May 26. Expert Opin Drug Discov. 2020. PMID: 32452701 Free PMC article. Review.
-
Multi-Targeting Bioactive Compounds Extracted from Essential Oils as Kinase Inhibitors.Molecules. 2020 May 6;25(9):2174. doi: 10.3390/molecules25092174. Molecules. 2020. PMID: 32384767 Free PMC article.
-
MTLD, a Database of Multiple Target Ligands, the Updated Version.Molecules. 2017 Sep 6;22(9):1375. doi: 10.3390/molecules22091375. Molecules. 2017. PMID: 28878188 Free PMC article.
-
LigAdvisor: a versatile and user-friendly web-platform for drug design.Nucleic Acids Res. 2021 Jul 2;49(W1):W326-W335. doi: 10.1093/nar/gkab385. Nucleic Acids Res. 2021. PMID: 34023895 Free PMC article.
-
Computational polypharmacology comes of age.Front Pharmacol. 2015 Jul 28;6:157. doi: 10.3389/fphar.2015.00157. eCollection 2015. Front Pharmacol. 2015. PMID: 26283966 Free PMC article. No abstract available.
References
-
- Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008, 411682–690. - PubMed
-
- Campillos M.; Kuhn M.; Gavin A. C.; Jensen L. J.; Bork P. Drug target identification using side-effect similarity. Science 2008, 3215886263–6. - PubMed
-
- Yildirim M. A.; Goh K. I.; Cusick M. E.; Barabasi A. L.; Vidal M. Drug-target network. Nat. Biotechnol. 2007, 25101119–26. - PubMed
-
- Xie L.; Xie L.; Kinnings S. L.; Bourne P. E. Novel Computational Approaches to Polypharmacology as a Means to Define Responses to Individual Drugs. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 361–379. - PubMed
-
- Keiser M. J.; Setola V.; Irwin J. J.; Laggner C.; Abbas A. I.; Hufeisen S. J.; Jensen N. H.; Kuijer M. B.; Matos R. C.; Tran T. B.; Whaley R.; Glennon R. A.; Hert J.; Thomas K. L. H.; Edwards D. D.; Shoichet B. K.; Roth B. L. Predicting new molecular targets for known drugs. Nature 2009, 4627270175–181. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

