Phylogenetic analyses and characterization of RNase X25 from Drosophila melanogaster suggest a conserved housekeeping role and additional functions for RNase T2 enzymes in protostomes
- PMID: 25133712
- PMCID: PMC4136927
- DOI: 10.1371/journal.pone.0105444
Phylogenetic analyses and characterization of RNase X25 from Drosophila melanogaster suggest a conserved housekeeping role and additional functions for RNase T2 enzymes in protostomes
Abstract
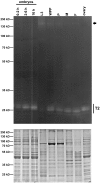
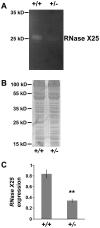
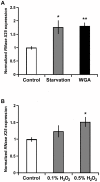
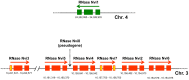
Ribonucleases belonging to the RNase T2 family are enzymes associated with the secretory pathway that are almost absolutely conserved in all eukaryotes. Studies in plants and vertebrates suggest they have an important housekeeping function in rRNA recycling. However, little is known about this family of enzymes in protostomes. We characterized RNase X25, the only RNase T2 enzyme in Drosophila melanogaster. We found that RNase X25 is the major contributor of ribonuclease activity in flies as detected by in gel assays, and has an acidic pH preference. Gene expression analyses showed that the RNase X25 transcript is present in all adult tissues and developmental stages. RNase X25 expression is elevated in response to nutritional stresses; consistent with the hypothesis that this enzyme has a housekeeping role in recycling RNA. A correlation between induction of RNase X25 expression and autophagy was observed. Moreover, induction of gene expression was triggered by oxidative stress suggesting that RNase X25 may have additional roles in stress responses. Phylogenetic analyses of this family in protostomes showed that RNase T2 genes have undergone duplication events followed by divergence in several phyla, including the loss of catalytic residues, and suggest that RNase T2 proteins have acquired novel functions. Among those, it is likely that a role in host immunosuppression evolved independently in several groups, including parasitic Platyhelminthes and parasitoid wasps. The presence of only one RNase T2 gene in the D. melanogaster genome, without any other evident secretory RNase activity detected, makes this organism an ideal system to study the cellular functions of RNase T2 proteins associated with RNA recycling and maintenance of cellular homeostasis. On the other hand, the discovery of gene duplications in several protostome genomes also presents interesting new avenues to study additional biological functions of this ancient family of proteins.
Conflict of interest statement
Figures






References
-
- Irie M (1999) Structure-function relationships of acid ribonucleases: lysosomal, vacuolar, and periplasmic enzymes. Pharmacol Ther 81: 77–89. - PubMed
-
- MacIntosh GC (2011) RNase T2 Family: Enzymatic Properties, Functional Diversity, and Evolution of Ancient Ribonucleases. In: Nicholson AWW, editor. Ribonucleases: Springer Berlin Heidelberg. pp. 89–114.
-
- Deshpande RA, Shankar V (2002) Ribonucleases from T2 family. Crit Rev Microbiol 28: 79–122. - PubMed
-
- Tanaka N, Arai J, Inokuchi N, Koyama T, Ohgi K, et al. (2000) Crystal structure of a plant ribonuclease, RNase LE. J Mol Biol 298: 859–873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

