Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial
- PMID: 25133822
- PMCID: PMC4312136
- DOI: 10.1037/hea0000088
Financial incentives for increasing uptake of HPV vaccinations: a randomized controlled trial
Abstract
Objective: Uptake of human papillomavirus (HPV) vaccinations by 17- to 18-year-old girls in England is below (<35%) target (80%). This trial assesses (a) the impact of financial incentives on uptake and completion of an HPV vaccination program, and (b) whether impacts are moderated by participants' deprivation level. It also assesses the impact of incentives on decision quality to get vaccinated, as measured by attitudes toward the vaccination and knowledge of its consequences.
Method: One thousand 16- to 18-year-old girls were invited to participate in an HPV vaccination program: 500 previously uninvited, and 500 unresponsive to previous invitations. Girls randomly received either a standard invitation letter or a letter including the offer of vouchers worth £ 45 (€ 56; $73) for undergoing 3 vaccinations. Girls attending their first vaccination appointment completed a questionnaire assessing decision quality to be vaccinated. Outcomes were uptake of the first and third vaccinations and decision quality.
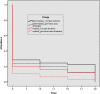
Results: The intervention increased uptake of the first (first-time invitees: 28.4% vs. 19.6%, odds ratio [OR] = 1.63, 95% confidence interval [CI; 1.08, 2.47]; previous nonattenders: 23.6% vs. 10.4%, OR = 2.65, 95% CI [1.61, 4.38]) and third (first-time invitees: 22.4% vs. 12%, OR = 2.15, 95% CI [1.32, 3.50]; previous nonattenders: 12.4% vs. 3%, OR = 4.28, 95% CI [1.92, 9.55]) vaccinations. Impacts were not moderated by deprivation level. Decision quality was unaffected by the intervention.
Conclusions: Although the intervention increased completion of HPV vaccinations, uptake remained lower than the national target, which, in addition to cost effectiveness and acceptability issues, necessitates consideration of other ways of achieving it.
Figures
References
-
- Ault K. A. (2007). Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: A combined analysis of four randomised clinical trials. The Lancet, 369, 1861–1868. doi:10.1016/S0140-6736(07)60852-6 - DOI - PubMed
-
- Balderson K. (2010). Girls bribed to take dangerous and pointless HPV vaccine. Retrieved fromhttp://wideshut.co.uk/girls-bribed-to-take-dangerous-and-pointless-hpv-v...
-
- Brabin L., Fairbrother E., Mandal D., Roberts S., Higgins S., Chandiok S., & Kitchener H. (2005). Biological and hormonal markers of chlamydia, human papillomavirus, and bacterial vaginosis among adolescents attending genitourinary medicine clinics. Sexually Transmitted Infections, 81, 128–132. doi:10.1136/sti.2004.010223 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources