Employment-based reinforcement of adherence to oral naltrexone in unemployed injection drug users: 12-month outcomes
- PMID: 25134047
- PMCID: PMC4339630
- DOI: 10.1037/adb0000010
Employment-based reinforcement of adherence to oral naltrexone in unemployed injection drug users: 12-month outcomes
Abstract
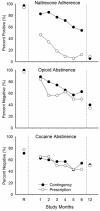
Oral naltrexone could be a promising relapse-prevention pharmacotherapy for recently detoxified opioid-dependent patients; however, interventions are often needed to promote adherence with this treatment approach. We recently conducted a study to evaluate a 26-week employment-based reinforcement intervention of oral naltrexone in unemployed injection drug users (Dunn et al., 2013). Participants were randomly assigned into a contingency (n = 35) group required to ingest naltrexone under staff observation to gain entry into a therapeutic workplace or a prescription (n = 32) group given a take-home supply of oral naltrexone and access to the workplace without observed ingestion. Monthly urine samples were collected and analyzed for evidence for naltrexone adherence, opioid use, and cocaine use. As previously reported, contingency participants provided significantly more naltrexone-positive urine samples than prescription participants during the 26-week intervention period. The goal of this current study is to report the 12-month outcomes, which occurred 6 months after the intervention ended. Results at the 12-month visit showed no between-groups differences in naltrexone-positive, opioid-negative, or cocaine-negative urine samples and no participant self-reported using naltrexone at the follow-up visit. These results show that even after a period of successfully reinforced oral naltrexone adherence, longer-term naltrexone use is unlikely to be maintained after reinforcement contingencies are discontinued. (PsycINFO Database Record
Trial registration: ClinicalTrials.gov NCT00149669.
(c) 2015 APA, all rights reserved).
Figures
References
-
- Adi Y, Juarez-Garcia A, Wang D, Jowett S, Frew E, Day E, et al. Oral naltrexone as a treatment for relapse prevention in formerly opioid-dependent drug users: A systematic review and economic evaluation. Health Technology Assessment (Winchester, England) 2007;11(6):iii–iv. 1-85. - PubMed
-
- Anton RF, Hogan I, Jalali B, Riordan CE, Kleber HD. Multiple family therapy and naltrexone in the treatment of opiate dependence. Drug and Alcohol Dependence. 1981;8(2):157–168. - PubMed
-
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI II) Psychology Corporation; San Antonio, TX: 1996.
-
- Broers B, Giner F, Dumont P, Mino A. Inpatient opiate detoxification in geneva: Follow-up at 1 and 6 months. Drug and Alcohol Dependence. 2000;58(1-2):85–92. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

