Amikacin pharmacokinetics during continuous veno-venous hemodialysis
- PMID: 25134484
- PMCID: PMC4108101
- DOI: 10.1007/s40121-013-0012-8
Amikacin pharmacokinetics during continuous veno-venous hemodialysis
Abstract
Introduction: Little is known about the pharmacokinetics of amikacin during continuous renal replacement therapy.
Methods: This prospective observational study included patients admitted to an academic medical center who received amikacin therapy while on continuous veno-venous hemodialysis (CVVHD) and had at least two serum sample concentrations measured after first-dose administration. First-order pharmacokinetic parameters, patient characteristics, and CVVHD parameters were recorded.
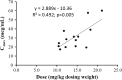
Results: Fifteen patients were included in the analysis. The median (interquartile range) dose of amikacin and dialysate flow rate, based on adjusted body weight, were 14.1 mg/kg (11.7-17.3 mg/kg) and 23.9 mL/kg/h (19.0-29.5 mL/kg/h), respectively. This corresponded with a median C max of 28.5 μg/mL (20.9-39.0 μg/mL). There was a significant correlation between clearance and dialytic dose (for every 1 L/h increase in dialysate flow rate, clearance rate increased by 23.6 mL/min [95% confidence interval 1.7-45.4 mL/min; P = 0.037]).
Conclusion: The results of this study suggest that amikacin dose and interval should be individualized for each patient on CVVHD based on first-dose pharmacokinetic assessment.
Similar articles
-
Comparative polymyxin B pharmacokinetics in critically ill patients with renal insufficiency and in continuous veno-venous hemodialysis.Eur J Clin Pharmacol. 2023 Jan;79(1):79-87. doi: 10.1007/s00228-022-03415-x. Epub 2022 Nov 15. Eur J Clin Pharmacol. 2023. PMID: 36378296
-
Influence of continuous veno-venous haemodiafiltration and continuous veno-venous haemofiltration on the pharmacokinetics of fluconazole.Eur J Clin Pharmacol. 2000 Dec;56(9-10):671-8. doi: 10.1007/s002280000216. Eur J Clin Pharmacol. 2000. PMID: 11214774 Clinical Trial.
-
Clearance of myoglobin by high cutoff continuous veno-venous hemodialysis in a patient with rhabdomyolysis: a case report.Hemodial Int. 2015 Jan;19(1):135-40. doi: 10.1111/hdi.12172. Epub 2014 Apr 27. Hemodial Int. 2015. PMID: 24766332
-
Determinants of Vancomycin Trough Concentration in Patients Receiving Continuous Veno-Venous Hemodialysis.Ann Pharmacother. 2022 Oct;56(10):1133-1138. doi: 10.1177/10600280211073370. Epub 2022 Feb 8. Ann Pharmacother. 2022. PMID: 35130750
-
Dosage adjustment of fluconazole during continuous renal replacement therapy (CAVH, CVVH, CAVHD, CVVHD).Mycoses. 1999 Apr;42(1-2):17-9. doi: 10.1046/j.1439-0507.1999.00269.x. Mycoses. 1999. PMID: 10394842 Review.
Cited by
-
How To Prescribe And Troubleshoot Continuous Renal Replacement Therapy: A Case-Based Review.Kidney360. 2020 Dec 14;2(2):371-384. doi: 10.34067/KID.0004912020. eCollection 2021 Feb 25. Kidney360. 2020. PMID: 35373031 Free PMC article. Review.
-
Amikacin use in critically ill patients requiring renal replacement therapy: the AMIDIAL-ICU study.Ann Intensive Care. 2025 Mar 26;15(1):42. doi: 10.1186/s13613-025-01461-z. Ann Intensive Care. 2025. PMID: 40133728 Free PMC article.
-
In vivo evaluation of drug dialyzability in a rat model of hemodialysis.PLoS One. 2020 Jun 12;15(6):e0233925. doi: 10.1371/journal.pone.0233925. eCollection 2020. PLoS One. 2020. PMID: 32530952 Free PMC article.
-
Influence of Renal Replacement Modalities on Amikacin Population Pharmacokinetics in Critically Ill Patients on Continuous Renal Replacement Therapy.Antimicrob Agents Chemother. 2016 Jul 22;60(8):4901-9. doi: 10.1128/AAC.00828-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27270279 Free PMC article. Clinical Trial.
-
Population Pharmacokinetic Study of the Suitability of Standard Dosing Regimens of Amikacin in Critically Ill Patients with Open-Abdomen and Negative-Pressure Wound Therapy.Antimicrob Agents Chemother. 2020 Mar 24;64(4):e02098-19. doi: 10.1128/AAC.02098-19. Print 2020 Mar 24. Antimicrob Agents Chemother. 2020. PMID: 31964795 Free PMC article.
References
-
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources