Evidence that meningeal mast cells can worsen stroke pathology in mice
- PMID: 25134760
- PMCID: PMC4188278
- DOI: 10.1016/j.ajpath.2014.06.003
Evidence that meningeal mast cells can worsen stroke pathology in mice
Abstract
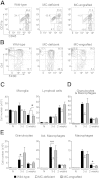
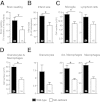
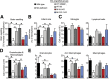
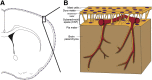
Stroke is the leading cause of adult disability and the fourth most common cause of death in the United States. Inflammation is thought to play an important role in stroke pathology, but the factors that promote inflammation in this setting remain to be fully defined. An understudied but important factor is the role of meningeal-located immune cells in modulating brain pathology. Although different immune cells traffic through meningeal vessels en route to the brain, mature mast cells do not circulate but are resident in the meninges. With the use of genetic and cell transfer approaches in mice, we identified evidence that meningeal mast cells can importantly contribute to the key features of stroke pathology, including infiltration of granulocytes and activated macrophages, brain swelling, and infarct size. We also obtained evidence that two mast cell-derived products, interleukin-6 and, to a lesser extent, chemokine (C-C motif) ligand 7, can contribute to stroke pathology. These findings indicate a novel role for mast cells in the meninges, the membranes that envelop the brain, as potential gatekeepers for modulating brain inflammation and pathology after stroke.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. - PMC - PubMed
-
- Donnan G.A., Fisher M., Macleod M., Davis S.M. Stroke. Lancet. 2008;371:1612–1623. - PubMed
-
- Macrez R., Ali C., Toutirais O., Le Mauff B., Defer G., Dirnagl U., Vivien D. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10:471–480. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

