Development of phage immuno-loop-mediated isothermal amplification assays for organophosphorus pesticides in agro-products
- PMID: 25135320
- PMCID: PMC4139188
- DOI: 10.1021/ac5020657
Development of phage immuno-loop-mediated isothermal amplification assays for organophosphorus pesticides in agro-products
Abstract
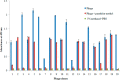
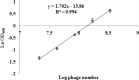
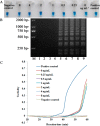
Two immuno-loop-mediated isothermal amplification assays (iLAMP) were developed by using a phage-borne peptide that was isolated from a cyclic eight-peptide phage library. One assay was used to screen eight organophosphorus (OP) pesticides with limits of detection (LOD) between 2 and 128 ng mL(-1). The iLAMP consisted of the competitive immuno-reaction coupled to the LAMP reaction for detection. This method provides positive results in the visual color of violet, while a negative response results in a sky blue color; therefore, the iLAMP allows one to rapidly detect analytes in yes or no fashion. We validated the iLAMP by detecting parathion-methyl, parathion, and fenitrothion in Chinese cabbage, apple, and greengrocery, and the detection results were consistent with the enzyme-linked immunosorbent assay (ELISA). In conclusion, the iLAMP is a simple, rapid, sensitive, and economical method for detecting OP pesticide residues in agro-products with no instrumental requirement.
Figures



Similar articles
-
Development of a heterologous enzyme-linked immunosorbent assay for organophosphorus pesticides with phage-borne peptide.RSC Adv. 2014 Jan 1;4(80):42445-42453. doi: 10.1039/C4RA07059C. RSC Adv. 2014. PMID: 26290688 Free PMC article.
-
Development of Anti-Idiotypic Nanobody-Phage Based Immuno-Loop-Mediated Isothermal Amplification Assay for Aflatoxins in Peanuts.Toxins (Basel). 2020 Sep 2;12(9):565. doi: 10.3390/toxins12090565. Toxins (Basel). 2020. PMID: 32887280 Free PMC article.
-
Development of a Mab-based heterologous immunoassay for the broad-selective determination of organophosphorus pesticides.J Agric Food Chem. 2010 May 12;58(9):5658-63. doi: 10.1021/jf904575k. J Agric Food Chem. 2010. PMID: 20297814
-
Development of a biotinylated broad-specificity single-chain variable fragment antibody and a sensitive immunoassay for detection of organophosphorus pesticides.Anal Bioanal Chem. 2016 Sep;408(23):6423-30. doi: 10.1007/s00216-016-9760-0. Epub 2016 Jul 13. Anal Bioanal Chem. 2016. PMID: 27411546
-
Bio-sensing of organophosphorus pesticides: A review.Biosens Bioelectron. 2019 Sep 1;140:111348. doi: 10.1016/j.bios.2019.111348. Epub 2019 May 24. Biosens Bioelectron. 2019. PMID: 31153016 Review.
Cited by
-
Recombinase polymerase amplification combined with a magnetic nanoparticle-based immunoassay for fluorometric determination of troponin T.Mikrochim Acta. 2019 Jul 18;186(8):549. doi: 10.1007/s00604-019-3686-0. Mikrochim Acta. 2019. PMID: 31321544
-
Sortase-Mediated Phage Decoration for Analytical Applications.Anal Chem. 2021 Aug 31;93(34):11800-11808. doi: 10.1021/acs.analchem.1c02322. Epub 2021 Aug 20. Anal Chem. 2021. PMID: 34415158 Free PMC article.
-
M13 phage: a versatile building block for a highly specific analysis platform.Anal Bioanal Chem. 2023 Jul;415(18):3927-3944. doi: 10.1007/s00216-023-04606-w. Epub 2023 Mar 3. Anal Bioanal Chem. 2023. PMID: 36867197 Free PMC article. Review.
-
Competitive and noncompetitive phage immunoassays for the determination of benzothiostrobin.Anal Chim Acta. 2015 Aug 26;890:150-6. doi: 10.1016/j.aca.2015.07.056. Epub 2015 Aug 13. Anal Chim Acta. 2015. PMID: 26347177 Free PMC article.
-
Magnetic-Immuno-Loop-Mediated Isothermal Amplification Based on DNA Encapsulating Liposome for the Ultrasensitive Detection of P-glycoprotein.Sci Rep. 2017 Aug 24;7(1):9312. doi: 10.1038/s41598-017-10133-3. Sci Rep. 2017. PMID: 28839228 Free PMC article.
References
-
- Roex E. W.; Keijzers R.; van Gestel C. A. Aquat. Toxicol. 2003, 64, 451–460. - PubMed
-
- Nagamine K.; Hase T.; Notomi T. Mol. Cell. Probes 2002, 16, 223–229. - PubMed
-
- Hara-Kudo Y.; Yoshino M.; Kojima T.; Ikedo M. FEMS Microbiol. Lett. 2005, 253, 155–161. - PubMed
-
- Hara-Kudo Y.; Nemoto J.; Ohtsuka K.; Segawa Y.; Takatori K.; Kojima T.; Ikedo M. J. Med. Microbiol. 2007, 56, 398–406. - PubMed
-
- Yamazaki W.; Taguchi M.; Ishibashi M.; Kitazato M.; Nukina M.; Misawa N.; Inoue K. J. Med. Microbiol. 2008, 57, 444–451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

