The tubulin code: molecular components, readout mechanisms, and functions
- PMID: 25135932
- PMCID: PMC4137062
- DOI: 10.1083/jcb.201406055
The tubulin code: molecular components, readout mechanisms, and functions
Abstract
Microtubules are cytoskeletal filaments that are dynamically assembled from α/β-tubulin heterodimers. The primary sequence and structure of the tubulin proteins and, consequently, the properties and architecture of microtubules are highly conserved in eukaryotes. Despite this conservation, tubulin is subject to heterogeneity that is generated in two ways: by the expression of different tubulin isotypes and by posttranslational modifications (PTMs). Identifying the mechanisms that generate and control tubulin heterogeneity and how this heterogeneity affects microtubule function are long-standing goals in the field. Recent work on tubulin PTMs has shed light on how these modifications could contribute to a "tubulin code" that coordinates the complex functions of microtubules in cells.
© 2014 Janke.
Figures
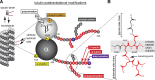

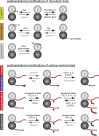

References
-
- Alexander, J.E., Hunt D.F., Lee M.K., Shabanowitz J., Michel H., Berlin S.C., MacDonald T.L., Sundberg R.J., Rebhun L.I., and Frankfurter A.. 1991. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc. Natl. Acad. Sci. USA. 88:4685–4689 10.1073/pnas.88.11.4685 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

