Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation
- PMID: 25135971
- PMCID: PMC4142031
- DOI: 10.1098/rstb.2013.0510
Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation
Abstract
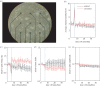
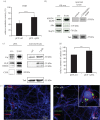
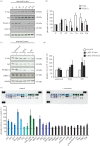
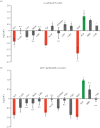
Exosomes are small membranous vesicles of endocytic origin that are released by almost every cell type. They exert versatile functions in intercellular communication important for many physiological and pathological processes. Recently, exosomes attracted interest with regard to their role in cell-cell communication in the nervous system. We have shown that exosomes released from oligodendrocytes upon stimulation with the neurotransmitter glutamate are internalized by neurons and enhance the neuronal stress tolerance. Here, we demonstrate that oligodendroglial exosomes also promote neuronal survival during oxygen-glucose deprivation, a model of cerebral ischaemia. We show the transfer from oligodendrocytes to neurons of superoxide dismutase and catalase, enzymes which are known to help cells to resist oxidative stress. Additionally, we identify various effects of oligodendroglial exosomes on neuronal physiology. Electrophysiological analysis using in vitro multi-electrode arrays revealed an increased firing rate of neurons exposed to oligodendroglial exosomes. Moreover, gene expression analysis and phosphorylation arrays uncovered differentially expressed genes and altered signal transduction pathways in neurons after exosome treatment. Our study thus provides new insight into the broad spectrum of action of oligodendroglial exosomes and their effects on neuronal physiology. The exchange of extracellular vesicles between neural cells may exhibit remarkable potential to impact brain performance.
Keywords: exosomes; extracellular vesicles; gene regulation; neuron–glia interaction; oligodendrocytes; signal transduction.
© 2014 The Author(s) Published by the Royal Society. All rights reserved.
Figures