Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes
- PMID: 25136078
- PMCID: PMC4358751
- DOI: 10.1161/CIRCRESAHA.115.304353
Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes
Abstract
Rationale: Bone marrow (BM) cell therapy for ischemic heart disease (IHD) has shown mixed results. Before the full potency of BM cell therapy can be realized, it is essential to understand the BM niche after acute myocardial infarction (AMI).
Objective: To study the BM composition in patients with IHD and severe left ventricular (LV) dysfunction.
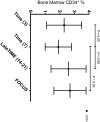
Methods and results: BM from 280 patients with IHD and LV dysfunction were analyzed for cell subsets by flow cytometry and colony assays. BM CD34(+) cell percentage was decreased 7 days after AMI (mean of 1.9% versus 2.3%-2.7% in other cohorts; P<0.05). BM-derived endothelial colonies were significantly decreased (P<0.05). Increased BM CD11b(+) cells associated with worse LV ejection fraction (LVEF) after AMI (P<0.05). Increased BM CD34(+) percentage associated with greater improvement in LVEF (+9.9% versus +2.3%; P=0.03, for patients with AMI and +6.6% versus -0.02%; P=0.021 for patients with chronic IHD). In addition, decreased BM CD34(+) percentage in patients with chronic IHD correlated with decrement in LVEF (-2.9% versus +0.7%; P=0.0355).
Conclusions: In this study, we show a heterogeneous mixture of BM cell subsets, decreased endothelial colony capacity, a CD34+ cell nadir 7 days after AMI, a negative correlation between CD11b percentage and postinfarct LVEF, and positive correlation of CD34 percentage with change in LVEF after cell therapy. These results serve as a possible basis for the small clinical improvement seen in autologous BM cell therapy trials and support selection of potent cell subsets and reversal of comorbid BM impairment.
Clinical trial registrations url: http://www.clinicaltrials.gov. Unique identifiers: NCT00684021, NCT00684060, and NCT00824005.
Keywords: angiogenesis effect; blood cells; bone marrow; myocardial infarction; stem cells.
© 2014 American Heart Association, Inc.
Conflict of interest statement
Figures






Comment in
-
Stem cell in the rough: repair quotient mined out of a bone marrow niche.Circ Res. 2014 Oct 24;115(10):814-6. doi: 10.1161/CIRCRESAHA.114.305075. Circ Res. 2014. PMID: 25342767 No abstract available.
-
Letter regarding the article, "a detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b, and clonogenic capacity as biomarkers for clinical outcomes".Circ Res. 2014 Dec 5;115(12):e35. doi: 10.1161/CIRCRESAHA.114.305312. Circ Res. 2014. PMID: 25477486 No abstract available.
-
Response to letter regarding article, "a detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b and clonogenic capacity as biomarkers for clinical outcomes".Circ Res. 2014 Dec 5;115(12):e36-7. doi: 10.1161/CIRCRESAHA.114.305422. Circ Res. 2014. PMID: 25477487 Free PMC article. No abstract available.
References
-
- Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. - PubMed
-
- Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37:1726–1732. - PubMed
-
- Madlambayan GJ, Butler JM, Hosaka K, Jorgensen M, Fu D, Guthrie SM, Shenoy AK, Brank A, Russell KJ, Otero J, Siemann DW, Scott EW, Cogle CR. Bone marrow stem and progenitor cell contribution to neovasculogenesis is dependent on model system with SDF-1 as a permissive trigger. Blood. 2009;114:4310–4319. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

