Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA)
- PMID: 25136079
- PMCID: PMC4145443
- DOI: 10.1136/bjophthalmol-2014-305702
Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA)
Abstract
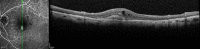
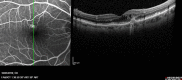
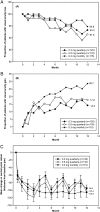
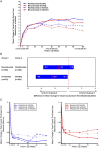
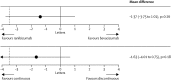
Age-related macular degeneration (AMD) is still referred to as the leading cause of severe and irreversible visual loss world-wide. The disease has a profound effect on quality of life of affected individuals and represents a major socioeconomic challenge for societies due to the exponential increase in life expectancy and environmental risks. Advances in medical research have identified vascular endothelial growth factor (VEGF) as an important pathophysiological player in neovascular AMD and intraocular inhibition of VEGF as one of the most efficient therapies in medicine. The wide introduction of anti-VEGF therapy has led to an overwhelming improvement in the prognosis of patients affected by neovascular AMD, allowing recovery and maintenance of visual function in the vast majority of patients. However, the therapeutic benefit is accompanied by significant economic investments, unresolved medicolegal debates about the use of off-label substances and overwhelming problems in large population management. The burden of disease has turned into a burden of care with a dissociation of scientific advances and real-world clinical performance. Simultaneously, ground-breaking innovations in diagnostic technologies, such as optical coherence tomography, allows unprecedented high-resolution visualisation of disease morphology and provides a promising horizon for early disease detection and efficient therapeutic follow-up. However, definite conclusions from morphologic parameters are still lacking, and valid biomarkers have yet to be identified to provide a practical base for disease management. The European Society of Retina Specialists offers expert guidance for diagnostic and therapeutic management of neovascular AMD supporting healthcare givers and doctors in providing the best state-of-the-art care to their patients.
Trial registration number: NCT01318941.
Keywords: Retina.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures
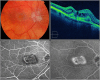
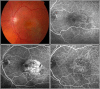

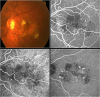

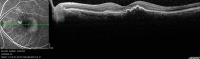



References
-
- Smith W, Assink J, Klein R, et al. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108:697–704 - PubMed
-
- Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology 2010;117:921–7 - PubMed
-
- Friedman DS, O'Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–72 - PubMed
-
- Seddon JM, Willett WC, Speizer FE, et al. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996;276: 1141–6 - PubMed
-
- Seddon JM, Cote J, Davis N, et al. Progression of age-related macular degeneration: association with body mass index, waist circumference, and waist-hip ratio. Arch Ophthalmol 2003;121:785–92 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
