IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells
- PMID: 25136113
- PMCID: PMC4156689
- DOI: 10.1073/pnas.1409781111
IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells
Abstract
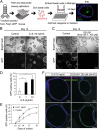
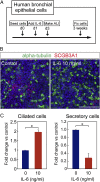
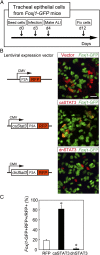
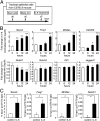
The pseudostratified airway epithelium of the lung contains a balanced proportion of multiciliated and secretory luminal cells that are maintained and regenerated by a population of basal stem cells. However, little is known about how these processes are modulated in vivo, and about the potential role of cytokine signaling between stem and progenitor cells and their niche. Using a clonal 3D organoid assay, we found that IL-6 stimulated, and Stat3 inhibitors reduced, the generation of ciliated vs. secretory cells from basal cells. Gain-of-function and loss-of-function studies with cultured mouse and human basal cells suggest that IL-6/Stat3 signaling promotes ciliogenesis at multiple levels, including increases in multicilin gene and forkhead box protein J1 expression and inhibition of the Notch pathway. To test the role of IL-6 in vivo genetically, we followed the regeneration of mouse tracheal epithelium after ablation of luminal cells by inhaled SO2. Stat3 is activated in basal cells and their daughters early in the repair process, correlating with an increase in Il-6 expression in platelet-derived growth factor receptor alpha(+) mesenchymal cells in the stroma. Conditional deletion in basal cells of suppressor of cytokine signaling 3, encoding a negative regulator of the Stat3 pathway, results in an increase in multiciliated cells at the expense of secretory and basal cells. By contrast, Il-6 null mice regenerate fewer ciliated cells and an increased number of secretory cells after injury. The results support a model in which IL-6, produced in the reparative niche, functions to enhance the differentiation of basal cells, and thereby acts as a "friend" to promote airway repair rather than a "foe."
Keywords: cell fate; epithelial repair; mucociliary epithelium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24(6):662–670. - PubMed
-
- Lai H, Rogers DF. New pharmacotherapy for airway mucus hypersecretion in asthma and COPD: Targeting intracellular signaling pathways. J Aerosol Med Pulm Drug Deliv. 2010;23(4):219–231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

