A sharp T-cell antigen receptor signaling threshold for T-cell proliferation
- PMID: 25136127
- PMCID: PMC4156735
- DOI: 10.1073/pnas.1413726111
A sharp T-cell antigen receptor signaling threshold for T-cell proliferation
Abstract
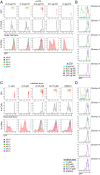
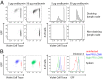
T-cell antigen receptor (TCR) signaling is essential for activation, proliferation, and effector function of T cells. Modulation of both intensity and duration of TCR signaling can regulate these events. However, it remains unclear how individual T cells integrate such signals over time to make critical cell-fate decisions. We have previously developed an engineered mutant allele of the critical T-cell kinase zeta-chain-associated protein kinase 70 kDa (Zap70) that is catalytically inhibited by a small molecule inhibitor, thereby blocking TCR signaling specifically and efficiently. We have also characterized a fluorescent reporter Nur77-eGFP transgenic mouse line in which T cells up-regulate GFP uniquely in response to TCR stimulation. The combination of these technologies unmasked a sharp TCR signaling threshold for commitment to cell division both in vitro and in vivo. Further, we demonstrate that this threshold is independent of both the magnitude of the TCR stimulus and Interleukin 2. Similarly, we identify a temporal threshold of TCR signaling that is required for commitment to proliferation, after which T cells are able to proliferate in a Zap70 kinase-independent manner. Taken together, our studies reveal a sharp threshold for the magnitude and duration of TCR signaling required for commitment of T cells to proliferation. These results have important implications for understanding T-cell responses to infection and optimizing strategies for immunomodulatory drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8(1):89–95. - PubMed
-
- Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419(6909):845–849. - PubMed
-
- Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172(10):6136–6143. - PubMed
-
- Kimachi K, Croft M, Grey HM. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur J Immunol. 1997;27(12):3310–3317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

