Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel
- PMID: 25136136
- PMCID: PMC4246943
- DOI: 10.1073/pnas.1408710111
Local anesthetic and antiepileptic drug access and binding to a bacterial voltage-gated sodium channel
Abstract
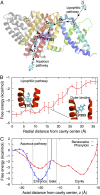
Voltage-gated sodium (Nav) channels are important targets in the treatment of a range of pathologies. Bacterial channels, for which crystal structures have been solved, exhibit modulation by local anesthetic and anti-epileptic agents, allowing molecular-level investigations into sodium channel-drug interactions. These structures reveal no basis for the "hinged lid"-based fast inactivation, seen in eukaryotic Nav channels. Thus, they enable examination of potential mechanisms of use- or state-dependent drug action based on activation gating, or slower pore-based inactivation processes. Multimicrosecond simulations of NavAb reveal high-affinity binding of benzocaine to F203 that is a surrogate for FS6, conserved in helix S6 of Domain IV of mammalian sodium channels, as well as low-affinity sites suggested to stabilize different states of the channel. Phenytoin exhibits a different binding distribution owing to preferential interactions at the membrane and water-protein interfaces. Two drug-access pathways into the pore are observed: via lateral fenestrations connecting to the membrane lipid phase, as well as via an aqueous pathway through the intracellular activation gate, despite being closed. These observations provide insight into drug modulation that will guide further developments of Nav inhibitors.
Keywords: bacterial sodium channel; drug binding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Mike A, Lukacs P. The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol. 2010;3(3):129–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

