Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria
- PMID: 25136281
- PMCID: PMC4127560
- DOI: 10.1186/1556-276X-9-373
Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria
Abstract
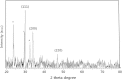
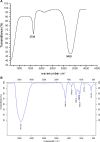
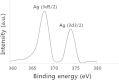
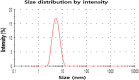
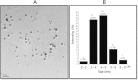
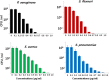
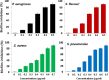
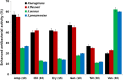
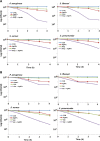
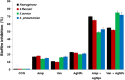
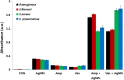
Silver nanoparticles (AgNPs) have been used as antibacterial, antifungal, antiviral, anti-inflammtory, and antiangiogenic due to its unique properties such as physical, chemical, and biological properties. The present study was aimed to investigate antibacterial and anti-biofilm activities of silver nanoparticles alone and in combination with conventional antibiotics against various human pathogenic bacteria. Here, we show that a simple, reliable, cost effective and green method for the synthesis of AgNPs by treating silver ions with leaf extract of Allophylus cobbe. The A. cobbe-mediated synthesis of AgNPs (AgNPs) was characterized by ultraviolet-visible absorption spectroscopy, X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), dynamic light scattering (DLS), and transmission electron microscopy (TEM). Furthermore, the antibacterial and anti-biofilm activity of antibiotics or AgNPs, or combinations of AgNPs with an antibiotic was evaluated using a series of assays: such as in vitro killing assay, disc diffusion assay, biofilm inhibition, and reactive oxygen species generation in Pseudomonas aeruginosa, Shigella flexneri, Staphylococcus aureus, and Streptococcus pneumonia. The results suggest that, in combination with antibiotics, there were significant antimicrobial and anti-biofilm effects at lowest concentration of AgNPs using a novel plant extract of A. cobbe, otherwise sublethal concentrations of the antibiotics. The significant enhancing effects were observed for ampicillin and vancomycin against Gram-negative and Gram-positive bacteria, respectively. These data suggest that combining antibiotics and biogenic AgNPs can be used therapeutically for the treatment of infectious diseases caused by bacteria. This study presented evidence of antibacterial and anti-biofilm effects of A. cobbe-mediated synthesis of AgNPs and their enhanced capacity against various human pathogenic bacteria. These results suggest that AgNPs could be used as an adjuvant for the treatment of infectious diseases.
Keywords: Allophylus cobbe; Anti-biofilm activity; Antibacterial activity; Antibiotics; Silver nanoparticles; Sublethal concentrations.
Figures
References
-
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;9:1–12. - PubMed
-
- Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, Tam PK, Chiu JF, Che CM. Silver nanoparticles: partial oxidation and antibacterial activities. J Biol Inorg Chem. 2007;9:527–534. - PubMed
-
- Malik MA, O'Brien P, Revaprasadu N. A simple route to the synthesis of core/shell nanoparticles of chalcogenides. Chem Mater. 2002;9:2004–2010.
-
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, Deepak V, Pandian SRK, Muniyandi J, Hariharan N, Eom SH. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloid Surf B. 2009;9:328–335. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

