Microtubule networks for plant cell division
- PMID: 25136380
- PMCID: PMC4127175
- DOI: 10.1007/s11693-014-9142-x
Microtubule networks for plant cell division
Abstract
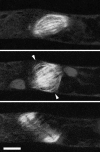
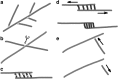
During cytokinesis the cytoplasm of a cell is divided to form two daughter cells. In animal cells, the existing plasma membrane is first constricted and then abscised to generate two individual plasma membranes. Plant cells on the other hand divide by forming an interior dividing wall, the so-called cell plate, which is constructed by localized deposition of membrane and cell wall material. Construction starts in the centre of the cell at the locus of the mitotic spindle and continues radially towards the existing plasma membrane. Finally the membrane of the cell plate and plasma membrane fuse to form two individual plasma membranes. Two microtubule-based cytoskeletal networks, the phragmoplast and the pre-prophase band (PPB), jointly control cytokinesis in plants. The bipolar microtubule array of the phragmoplast regulates cell plate deposition towards a cortical position that is templated by the ring-shaped microtubule array of the PPB. In contrast to most animal cells, plants do not use centrosomes as foci of microtubule growth initiation. Instead, plant microtubule networks are striking examples of self-organizing systems that emerge from physically constrained interactions of dispersed microtubules. Here we will discuss how microtubule-based activities including growth, shrinkage, severing, sliding, nucleation and bundling interrelate to jointly generate the required ordered structures. Evidence mounts that adapter proteins sense the local geometry of microtubules to locally modulate the activity of proteins involved in microtubule growth regulation and severing. Many of the proteins and mechanisms involved have roles in other microtubule assemblies as well, bestowing broader relevance to insights gained from plants.
Keywords: Cortical array; Cytokinesis; Cytoskeleton; Microtubule; Phragmoplast; Plant.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

