Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase
- PMID: 25136404
- PMCID: PMC4127243
- DOI: 10.1155/2014/893516
Anandamide protects HT22 cells exposed to hydrogen peroxide by inhibiting CB1 receptor-mediated type 2 NADPH oxidase
Abstract
Background: Endogenous cannabinoid anandamide (AEA) protects neurons from oxidative injury in rodent models; however the mechanism of AEA-induced neuroprotection remains to be determined. Activation of neuronal NADPH oxidase 2 (Nox2) contributes to oxidative damage of the brain, and inhibition of Nox2 can attenuate cerebral oxidative stress. We aimed to determine whether the neuronal Nox2 was involved in protection mediated by AEA.
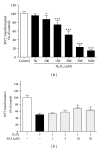
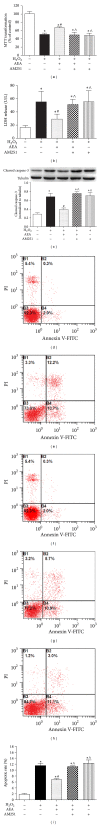
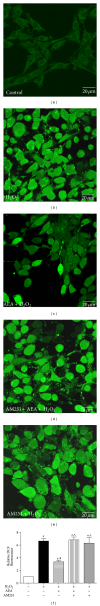
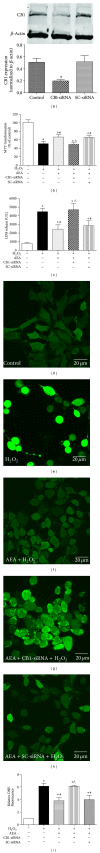
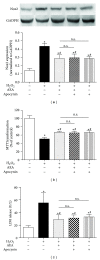
Methods: The mouse hippocampal neuron cell line HT22 was exposed to hydrogen peroxide (H2O2) to mimic oxidative injury of neurons. The protective effect of AEA was assessed by measuring cell metabolic activity, apoptosis, lactate dehydrogenase (LDH) release, cellular morphology, intracellular reactive oxygen species (ROS), and antioxidant and oxidant levels and Nox2 expression.
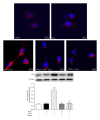
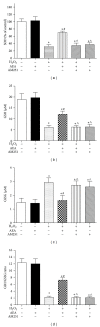
Results: HT22 cells exposed to H2O2 demonstrated morphological changes, decreased LDH release, reduced metabolic activity, increased levels of intracellular ROS and oxidized glutathione (GSSG), reduced levels of superoxide dismutase (SOD), and reduced glutathione (GSH) and increased expression of Nox2. AEA prevented these effects, a property abolished by simultaneous administration of CB1 antagonist AM251 or CB1-siRNA.
Conclusion: Nox2 inhibition is involved in AEA-induced cytoprotection against oxidative stress through CB1 activation in HT22 cells.
Figures



Similar articles
-
N‑linoleyltyrosine protects PC12 cells against oxidative damage via autophagy: Possible involvement of CB1 receptor regulation.Int J Mol Med. 2020 Nov;46(5):1827-1837. doi: 10.3892/ijmm.2020.4706. Epub 2020 Aug 24. Int J Mol Med. 2020. PMID: 33000188 Free PMC article.
-
Inhibitions of anandamide transport and FAAH synthesis decrease apoptosis and oxidative stress through inhibition of TRPV1 channel in an in vitro seizure model.Mol Cell Biochem. 2019 Mar;453(1-2):143-155. doi: 10.1007/s11010-018-3439-0. Epub 2018 Aug 29. Mol Cell Biochem. 2019. PMID: 30159798
-
The major endocannabinoid anandamide (AEA) induces apoptosis of human granulosa cells.Prostaglandins Leukot Essent Fatty Acids. 2021 Aug;171:102311. doi: 10.1016/j.plefa.2021.102311. Epub 2021 Jun 8. Prostaglandins Leukot Essent Fatty Acids. 2021. PMID: 34126378
-
Role of microglia in oxidative toxicity associated with encephalomycarditis virus infection in the central nervous system.Int J Mol Sci. 2012;13(6):7365-7374. doi: 10.3390/ijms13067365. Epub 2012 Jun 14. Int J Mol Sci. 2012. PMID: 22837699 Free PMC article. Review.
-
Oxidative stress, redox signalling and endothelial dysfunction in ageing-related neurodegenerative diseases: a role of NADPH oxidase 2.Br J Clin Pharmacol. 2014 Sep;78(3):441-53. doi: 10.1111/bcp.12357. Br J Clin Pharmacol. 2014. PMID: 25279404 Free PMC article. Review.
Cited by
-
Modulation of cellular redox homeostasis by the endocannabinoid system.Open Biol. 2016 Apr;6(4):150276. doi: 10.1098/rsob.150276. Epub 2016 Apr 27. Open Biol. 2016. PMID: 27248801 Free PMC article. Review.
-
A Single δ9-Tetrahydrocannabinol (THC) Dose During Brain Development Affects Markers of Neurotrophy, Oxidative Stress, and Apoptosis.Front Pharmacol. 2019 Oct 4;10:1156. doi: 10.3389/fphar.2019.01156. eCollection 2019. Front Pharmacol. 2019. PMID: 31636565 Free PMC article.
-
SOD2 Mediates Curcumin-Induced Protection against Oxygen-Glucose Deprivation/Reoxygenation Injury in HT22 Cells.Evid Based Complement Alternat Med. 2019 Sep 29;2019:2160642. doi: 10.1155/2019/2160642. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 31662771 Free PMC article.
-
N‑linoleyltyrosine protects PC12 cells against oxidative damage via autophagy: Possible involvement of CB1 receptor regulation.Int J Mol Med. 2020 Nov;46(5):1827-1837. doi: 10.3892/ijmm.2020.4706. Epub 2020 Aug 24. Int J Mol Med. 2020. PMID: 33000188 Free PMC article.
-
Inhibition of Monoacylglycerol Lipase by NSD1819 as an Effective Strategy for the Endocannabinoid System Modulation against Neuroinflammation-Related Disorders.Int J Mol Sci. 2022 Jul 29;23(15):8428. doi: 10.3390/ijms23158428. Int J Mol Sci. 2022. PMID: 35955562 Free PMC article.
References
-
- Gheysarzadeh A, Yazdanparast R. Inhibition of H2O2-induced cell death through FOXO1 modulation by EUK-172 in SK-N-MC cells. European Journal of Pharmacology. 2012;697(1–3):47–52. - PubMed
-
- Lu X, Xu H, Sun B, Zhu Z, Zheng D, Li X. Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Molecular Pharmaceutics. 2013;10(5):2045–2053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

