Selective HDAC inhibition for the disruption of latent HIV-1 infection
- PMID: 25136952
- PMCID: PMC4138023
- DOI: 10.1371/journal.pone.0102684
Selective HDAC inhibition for the disruption of latent HIV-1 infection
Abstract
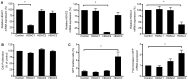
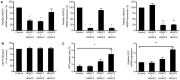
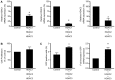
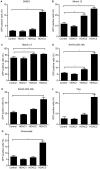
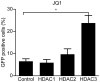
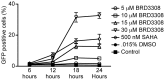
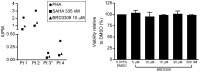
Selective histone deacetylase (HDAC) inhibitors have emerged as a potential anti-latency therapy for persistent human immunodeficiency virus type 1 (HIV-1) infection. We utilized a combination of small molecule inhibitors and short hairpin (sh)RNA-mediated gene knockdown strategies to delineate the key HDAC(s) to be targeted for selective induction of latent HIV-1 expression. Individual depletion of HDAC3 significantly induced expression from the HIV-1 promoter in the 2D10 latency cell line model. However, depletion of HDAC1 or -2 alone or in combination did not significantly induce HIV-1 expression. Co-depletion of HDAC2 and -3 resulted in a significant increase in expression from the HIV-1 promoter. Furthermore, concurrent knockdown of HDAC1, -2, and -3 resulted in a significant increase in expression from the HIV-1 promoter. Using small molecule HDAC inhibitors of differing selectivity to ablate the residual HDAC activity that remained after (sh)RNA depletion, the effect of depletion of HDAC3 was further enhanced. Enzymatic inhibition of HDAC3 with the selective small-molecule inhibitor BRD3308 activated HIV-1 transcription in the 2D10 cell line. Furthermore, ex vivo exposure to BRD3308 induced outgrowth of HIV-1 from resting CD4+ T cells isolated from antiretroviral-treated, aviremic HIV+ patients. Taken together these findings suggest that HDAC3 is an essential target to disrupt HIV-1 latency, and inhibition of HDAC2 may also contribute to the effort to purge and eradicate latent HIV-1 infection.
Conflict of interest statement
Figures
References
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, et al. (1997) Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278: 1295–1300. - PubMed
-
- Finzi D, Blankson J, Siliciano JD, Margolick JB, Chadwick K, et al. (1999) Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5: 512–517. - PubMed
-
- UNAIDS (2008) 2008 Report on the global AIDS epidemic.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

