Hydrogen bonding networks tune proton-coupled redox steps during the enzymatic six-electron conversion of nitrite to ammonia
- PMID: 25137350
- PMCID: PMC4159211
- DOI: 10.1021/bi500854p
Hydrogen bonding networks tune proton-coupled redox steps during the enzymatic six-electron conversion of nitrite to ammonia
Abstract
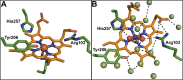
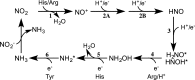
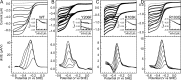
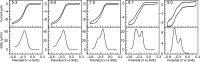
Multielectron multiproton reactions play an important role in both biological systems and chemical reactions involved in energy storage and manipulation. A key strategy employed by nature in achieving such complex chemistry is the use of proton-coupled redox steps. Cytochrome c nitrite reductase (ccNiR) catalyzes the six-electron seven-proton reduction of nitrite to ammonia. While a catalytic mechanism for ccNiR has been proposed on the basis of studies combining computation and crystallography, there have been few studies directly addressing the nature of the proton-coupled events that are predicted to occur along the nitrite reduction pathway. Here we use protein film voltammetry to directly interrogate the proton-coupled steps that occur during nitrite reduction by ccNiR. We find that conversion of nitrite to ammonia by ccNiR adsorbed to graphite electrodes is defined by two distinct phases; one is proton-coupled, and the other is not. Mutation of key active site residues (H257, R103, and Y206) modulates these phases and specifically alters the properties of the detected proton-dependent step but does not inhibit the ability of ccNiR to conduct the full reduction of nitrite to ammonia. We conclude that the active site residues examined are responsible for tuning the protonation steps that occur during catalysis, likely through an extensive hydrogen bonding network, but are not necessarily required for the reaction to proceed. These results provide important insight into how enzymes can specifically tune proton- and electron transfer steps to achieve high turnover numbers in a physiological pH range.
Figures

References
-
- Crane B. R.; Siegel L. M.; Getzoff E. D. (1997) Probing the catalytic mechanism of sulfite reductase by X-ray crystallography: Structures of the Escherichia coli hemoprotein in complex with substrates, inhibitors, intermediates, and products. Biochemistry 36, 12120–12137. - PubMed
-
- Gagliardi C. J.; Westlake B. C.; Kent C. A.; Paul J. J.; Papanikolas J. M.; Meyer T. J. (2010) Integrating proton coupled electron transfer (PCET) and excited states. Coord. Chem. Rev. 254, 2459–2471.
-
- Weinberg D. R.; Gagliardi C. J.; Hull J. F.; Murphy C. F.; Kent C. A.; Westlake B. C.; Paul A.; Ess D. H.; McCafferty D. G.; Meyer T. J. (2012) Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

