The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function
- PMID: 25137456
- PMCID: PMC4348363
- DOI: 10.1038/ni.2938
The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function
Abstract
Self-reactivity was once seen as a potential characteristic of T cells that was eliminated by clonal selection to protect the host from autoimmune pathology. It is now understood that the T cell repertoire is in fact broadly self-reactive, even self-centered. The strength with which a T cell reacts to self ligands and the environmental context in which this reaction occurs influence almost every aspect of T cell biology, from development to differentiation to effector function. Here we highlight recent advances and discoveries that relate to T cell self-reactivity, with a particular emphasis on T cell antigen receptor (TCR) signaling thresholds.
Figures
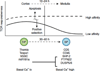
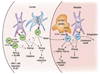
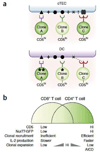
References
-
- Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nature reviews Immunology. 2005;5(10):772–782. - PubMed
-
-
Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, Mueller DL, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(12):4679–4684. Using Bim deficient Nur77GFP reporter mice, this study reported that the extent of negative selection is far greater than previously appreciated.
-
-
-
Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. The Journal of experimental medicine. 2013;210(2):269–285. This study reports that helios expression distinguishes cells undergoing positive and negative selection in the thymus. Analogous to the previous study, they analyzed helios expresion in Bim deficient mice to define the extent of clonal deletion.
-
-
-
Sinclair C, Bains I, Yates AJ, Seddon B. Asymmetric thymocyte death underlies the CD4:CD8 T-cell ratio in the adaptive immune system. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(31):E2905–E2914. Sinclair and colleagues estimated rates of death and differentiation using mathematical analysis of synchronized cohorts of thymocytes developing in an inducible ZAP70 model. Their results suggested an asymmetry in the death rates of Class I and Class II restricted thymocytes, and concurred remarkably well with the previous two studies that the majority of cells that start selection fail to complete it.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

