Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents
- PMID: 25137629
- PMCID: PMC4324418
- DOI: 10.1021/cn500128b
Selective activation of M4 muscarinic acetylcholine receptors reverses MK-801-induced behavioral impairments and enhances associative learning in rodents
Abstract
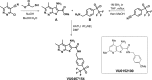
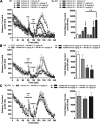
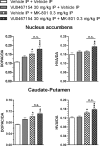
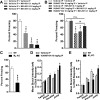
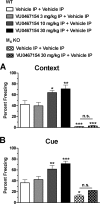
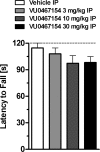
Positive allosteric modulators (PAMs) of the M4 muscarinic acetylcholine receptor (mAChR) represent a novel approach for the treatment of psychotic symptoms associated with schizophrenia and other neuropsychiatric disorders. We recently reported that the selective M4 PAM VU0152100 produced an antipsychotic drug-like profile in rodents after amphetamine challenge. Previous studies suggest that enhanced cholinergic activity may also improve cognitive function and reverse deficits observed with reduced signaling through the N-methyl-d-aspartate subtype of the glutamate receptor (NMDAR) in the central nervous system. Prior to this study, the M1 mAChR subtype was viewed as the primary candidate for these actions relative to the other mAChR subtypes. Here we describe the discovery of a novel M4 PAM, VU0467154, with enhanced in vitro potency and improved pharmacokinetic properties relative to other M4 PAMs, enabling a more extensive characterization of M4 actions in rodent models. We used VU0467154 to test the hypothesis that selective potentiation of M4 receptor signaling could ameliorate the behavioral, cognitive, and neurochemical impairments induced by the noncompetitive NMDAR antagonist MK-801. VU0467154 produced a robust dose-dependent reversal of MK-801-induced hyperlocomotion and deficits in preclinical models of associative learning and memory functions, including the touchscreen pairwise visual discrimination task in wild-type mice, but failed to reverse these stimulant-induced deficits in M4 KO mice. VU0467154 also enhanced the acquisition of both contextual and cue-mediated fear conditioning when administered alone in wild-type mice. These novel findings suggest that M4 PAMs may provide a strategy for addressing the more complex affective and cognitive disruptions associated with schizophrenia and other neuropsychiatric disorders.
Keywords: Allosteric modulator; M4 muscarinic; MK-801; VU0467154; antipsychotic; cognitive enhancement.
Figures







References
-
- Poels E. M.; Kegeles L. S.; Kantrowitz J. T.; Slifstein M.; Javitt D. C.; Lieberman J. A.; Abi-Dargham A.; Girgis R. R. (2014) Imaging glutamate in schizophrenia: Review of findings and implications for drug discovery. Mol. Psychiatry 19, 20–29. - PubMed
-
- Coyle J. T.; Tsai G. (2004) NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int. Rev. Neurobiol. 59, 491–515. - PubMed
-
- Rezvani A. H. (2006) Involvement of the NMDA System in Learning and Memory, In Animal Models of Cognitive Impairment (Levin E. D., and Buccafusco J. J., Eds.), pp 37–48, CRC Press, Boca Raton, Florida, U.S.A. - PubMed
-
- Bubser M.; Byun N.; Wood M. R.; Jones C. K. (2012) Muscarinic receptor pharmacology and circuitry for the modulation of cognition. Handb. Exp. Pharmacol. 121–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

