Chiral amine synthesis using ω-transaminases: an amine donor that displaces equilibria and enables high-throughput screening
- PMID: 25138082
- PMCID: PMC4497610
- DOI: 10.1002/anie.201406571
Chiral amine synthesis using ω-transaminases: an amine donor that displaces equilibria and enables high-throughput screening
Abstract
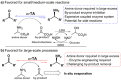
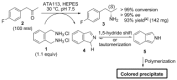
The widespread application of ω-transaminases as biocatalysts for chiral amine synthesis has been hampered by fundamental challenges, including unfavorable equilibrium positions and product inhibition. Herein, an efficient process that allows reactions to proceed in high conversion in the absence of by-product removal using only one equivalent of a diamine donor (ortho-xylylenediamine) is reported. This operationally simple method is compatible with the most widely used (R)- and (S)-selective ω-TAs and is particularly suitable for the conversion of substrates with unfavorable equilibrium positions (e.g., 1-indanone). Significantly, spontaneous polymerization of the isoindole by-product generates colored derivatives, providing a high-throughput screening platform to identify desired ω-TA activity.
Keywords: asymmetric catalysis; biocatalysis; chiral amines; high-throughput screening; transaminases.
© 2014 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures

Similar articles
-
A New Generation of Smart Amine Donors for Transaminase-Mediated Biotransformations.Chemistry. 2016 Aug 26;22(36):12692-5. doi: 10.1002/chem.201603188. Epub 2016 Aug 3. Chemistry. 2016. PMID: 27411957
-
Transaminases for chiral amine synthesis.Curr Opin Chem Biol. 2018 Apr;43:106-112. doi: 10.1016/j.cbpa.2017.12.007. Epub 2018 Jan 4. Curr Opin Chem Biol. 2018. PMID: 29278779 Review.
-
Amine transaminases in chiral amines synthesis: recent advances and challenges.World J Microbiol Biotechnol. 2017 Dec 18;34(1):13. doi: 10.1007/s11274-017-2395-2. World J Microbiol Biotechnol. 2017. PMID: 29255954 Review.
-
Process considerations for the asymmetric synthesis of chiral amines using transaminases.Biotechnol Bioeng. 2011 Jul;108(7):1479-93. doi: 10.1002/bit.23154. Epub 2011 Apr 14. Biotechnol Bioeng. 2011. PMID: 21455931 Review.
-
In vivo plug-and-play: a modular multi-enzyme single-cell catalyst for the asymmetric amination of ketoacids and ketones.Microb Cell Fact. 2017 Jul 28;16(1):132. doi: 10.1186/s12934-017-0750-5. Microb Cell Fact. 2017. PMID: 28754115 Free PMC article.
Cited by
-
CO2-Assisted asymmetric hydrogenation of prochiral allylamines.RSC Adv. 2022 Feb 28;12(11):6755-6761. doi: 10.1039/d2ra00263a. eCollection 2022 Feb 22. RSC Adv. 2022. PMID: 35424608 Free PMC article.
-
A Quantum Mechanical Approach to The Mechanism of Asymmetric Synthesis of Chiral Amine by Imine Reductase from Stackebrandtia Nassauensis.Chempluschem. 2025 Jan;90(1):e202400606. doi: 10.1002/cplu.202400606. Epub 2024 Nov 15. Chempluschem. 2025. PMID: 39434680 Free PMC article.
-
Synthesis of Sitagliptin Intermediate by a Multi-Enzymatic Cascade System Using Lipase and Transaminase With Benzylamine as an Amino Donor.Front Bioeng Biotechnol. 2021 Oct 6;9:757062. doi: 10.3389/fbioe.2021.757062. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34692666 Free PMC article.
-
Identification of (S)-selective transaminases for the asymmetric synthesis of bulky chiral amines.Nat Chem. 2016 Nov;8(11):1076-1082. doi: 10.1038/nchem.2578. Epub 2016 Jul 18. Nat Chem. 2016. PMID: 27768108
-
Droplet Microfluidics and Directed Evolution of Enzymes: An Intertwined Journey.Angew Chem Int Ed Engl. 2021 Nov 8;60(46):24368-24387. doi: 10.1002/anie.202016154. Epub 2021 Jul 16. Angew Chem Int Ed Engl. 2021. PMID: 33539653 Free PMC article. Review.
References
-
- Constable DJC. Green Chem. 2007;9:411. et al.,
-
- p. 347.
-
- Cassiano NM. Alkaloids: Properties, Applications and Pharmacological Effect. New York: Nova Science Pub. Inc;
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

