Rapid binding of plasminogen to streptokinase in a catalytic complex reveals a three-step mechanism
- PMID: 25138220
- PMCID: PMC4183831
- DOI: 10.1074/jbc.M114.589077
Rapid binding of plasminogen to streptokinase in a catalytic complex reveals a three-step mechanism
Abstract
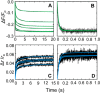
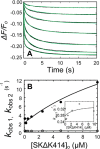
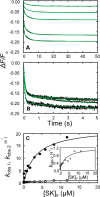
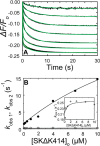
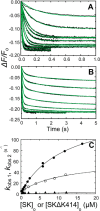
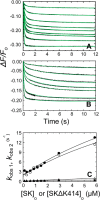
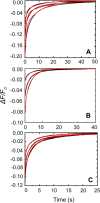
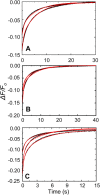
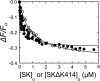
Rapid kinetics demonstrate a three-step pathway of streptokinase (SK) binding to plasminogen (Pg), the zymogen of plasmin (Pm). Formation of a fluorescently silent encounter complex is followed by two conformational tightening steps reported by fluorescence quenches. Forward reactions were defined by time courses of biphasic quenching during complex formation between SK or its COOH-terminal Lys(414) deletion mutant (SKΔK414) and active site-labeled [Lys]Pg ([5-(acetamido)fluorescein]-D-Phe-Phe-Arg-[Lys]Pg ([5F]FFR-[Lys]Pg)) and by the SK dependences of the quench rates. Active site-blocked Pm rapidly displaced [5F]FFR-[Lys]Pg from the complex. The encounter and final SK ·[5F]FFR-[Lys]Pg complexes were weakened similarly by SK Lys(414) deletion and blocking of lysine-binding sites (LBSs) on Pg kringles with 6-aminohexanoic acid or benzamidine. Forward and reverse rates for both tightening steps were unaffected by 6-aminohexanoic acid, whereas benzamidine released constraints on the first conformational tightening. This indicated that binding of SK Lys(414) to Pg kringle 4 plays a role in recognition of Pg by SK. The substantially lower affinity of the final SK · Pg complex compared with SK · Pm is characterized by a ∼ 25-fold weaker encounter complex and ∼ 40-fold faster off-rates for the second conformational step. The results suggest that effective Pg encounter requires SK Lys(414) engagement and significant non-LBS interactions with the protease domain, whereas Pm binding additionally requires contributions of other lysines. This difference may be responsible for the lower affinity of the SK · Pg complex and the expression of a weaker "pro"-exosite for binding of a second Pg in the substrate mode compared with SK · Pm.
Keywords: Bacterial Pathogenesis; Fibrinolysis; Fluorescence; Kinetics; Plasmin; Streptokinase.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

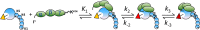
Similar articles
-
Pathogen activators of plasminogen.J Thromb Haemost. 2015 Jun;13 Suppl 1(0 1):S106-14. doi: 10.1111/jth.12939. J Thromb Haemost. 2015. PMID: 26149011 Free PMC article. Review.
-
Plasminogen substrate recognition by the streptokinase-plasminogen catalytic complex is facilitated by Arg253, Lys256, and Lys257 in the streptokinase beta-domain and kringle 5 of the substrate.J Biol Chem. 2009 Jul 17;284(29):19511-21. doi: 10.1074/jbc.M109.005512. Epub 2009 May 27. J Biol Chem. 2009. PMID: 19473980 Free PMC article.
-
Binding of the COOH-terminal lysine residue of streptokinase to plasmin(ogen) kringles enhances formation of the streptokinase.plasmin(ogen) catalytic complexes.J Biol Chem. 2006 Sep 15;281(37):26774-8. doi: 10.1074/jbc.C600171200. Epub 2006 Jul 20. J Biol Chem. 2006. PMID: 16857686 Free PMC article.
-
Rapid-reaction kinetic characterization of the pathway of streptokinase-plasmin catalytic complex formation.J Biol Chem. 2008 Sep 19;283(38):26137-47. doi: 10.1074/jbc.M804038200. Epub 2008 Jul 25. J Biol Chem. 2008. PMID: 18658146 Free PMC article.
-
Species specificity of streptokinase.Comp Biochem Physiol B. 1983;75(3):389-94. doi: 10.1016/0305-0491(83)90345-0. Comp Biochem Physiol B. 1983. PMID: 6349918 Review.
Cited by
-
Lysine Residues in the MK-Rich Region Are Not Required for Binding of the PbsP Protein From Group B Streptococci to Plasminogen.Front Cell Infect Microbiol. 2021 Sep 8;11:679792. doi: 10.3389/fcimb.2021.679792. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34568085 Free PMC article.
-
Pathogen activators of plasminogen.J Thromb Haemost. 2015 Jun;13 Suppl 1(0 1):S106-14. doi: 10.1111/jth.12939. J Thromb Haemost. 2015. PMID: 26149011 Free PMC article. Review.
-
Design of a DNA-Programmed Plasminogen Activator.J Am Chem Soc. 2018 Nov 14;140(45):15516-15524. doi: 10.1021/jacs.8b10166. Epub 2018 Nov 1. J Am Chem Soc. 2018. PMID: 30347143 Free PMC article.
-
Targeted drug delivery to the thrombus by fusing streptokinase with a fibrin-binding peptide (CREKA): an in silico study.Ther Deliv. 2024;15(6):399-411. doi: 10.4155/tde-2023-0107. Epub 2024 Apr 30. Ther Deliv. 2024. PMID: 38686829 Free PMC article.
-
Lactoferrin is a natural inhibitor of plasminogen activation.J Biol Chem. 2018 Jun 1;293(22):8600-8613. doi: 10.1074/jbc.RA118.003145. Epub 2018 Apr 18. J Biol Chem. 2018. PMID: 29669808 Free PMC article.
References
-
- Collen D., Lijnen H. R. (1995) Molecular basis of fibrinolysis, as relevant for thrombolytic therapy. Thromb. Haemost. 74, 167–171 - PubMed
-
- Carapetis J. R., Steer A. C., Mulholland E. K., Weber M. (2005) The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5, 685–694 - PubMed
-
- Lopardo H. A., Vidal P., Sparo M., Jeric P., Centron D., Facklam R. R., Paganini H., Pagniez N. G., Lovgren M., Beall B. (2005) Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J. Clin. Microbiol. 43, 802–807 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

