Coupling of sterically hindered trisubstituted olefins and benzocyclobutenones by C-C activation: total synthesis and structural revision of cycloinumakiol
- PMID: 25138969
- PMCID: PMC4214140
- DOI: 10.1002/anie.201404802
Coupling of sterically hindered trisubstituted olefins and benzocyclobutenones by C-C activation: total synthesis and structural revision of cycloinumakiol
Abstract
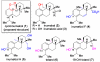
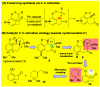
The first total syntheses of the proposed structure of cycloinumakiol (1) and its C5 epimer (18) are achieved in a concise and efficient fashion. Starting from the known 3-hydroxybenzocyclobutenone, 1 and 18 are obtained in nine and five steps with overall yields of 15% and 33%, respectively. The key for the success of this approach is the use of a catalytic C-C activation strategy for constructing the tetracyclic core of 1 through carboacylation of a sterically hindered trisubstituted olefin with benzocyclobutenone. In addition, the structure of the natural cycloinumakiol was reassigned to 19-hydroxytotarol (7) through X-ray diffraction analysis. This work demonstrates the potential of C-C activation for streamlining complex natural product synthesis.
Keywords: CC activation; Rh catalysis; cycloinumakiol; structure elucidation; total synthesis.
© 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures





References
-
-
Sato K, et al. Chem. Pharm. Bull. 2008;56:1691–1697. for a review of totarol, see: Bendall JG, Cambie RC. Aust. J. Chem. 1995;48:883–917. Park H-S, Takahashi Y, Fukaya H, Aoyagi Y, Takeya K. J. Nat. Prod. 2003;66:282–284. Park H-S, Yoda N, Fukaya H, Aoyagi Y, Takeya K. Tetrahedron. 2004;60:171–177.
-
-
-
For recent total synthesis of totarols, see: Barltrop JA, Rogers NAJ. J. Chem. Soc. 1958:2566–2572. Tada M, Kurabe J, Yasue H, Ikuta T. Chem. Pharm. Bull. 2008;56:287–291. Kim MB, Shaw JT. Org. Lett. 2010;12:3324–3327.
-
-
-
Xu T, Dong G. Angew. Chem. Int. Ed. 2012;51:7567–7571. Xu T, Ko HM, Savage NA, Dong G. J. Am. Chem. Soc. 2012;134:20005–20008. for alkyne insertion, see: Chen P, Xu T, Dong G. Angew. Chem. Int. Ed. 2014;53:1674–1678.
-
-
-
For selected reviews on transition metal-mediated C-C bond activation, see: Jones WD. Nature. 1993;364:676–677. Murakami M, Ito Y. Top. Organomet. Chem. 1999;3:97–129. Rybtchiski B, Milstein D. Angew. Chem. Int. Ed. 1999;38:870–883. Perthuisot C, Edelbach BL, Zubris DL, Simhai N, Iverson CN, Müller C, Satoh T, Jones WD. J. Catal. Mol. A. 2002;189:157–168. van der Boom ME, Milstein D. Chem. Rev. 2003;103:1759–1792. Jun C-H. Chem. Soc. Rev. 2004;33:610–618. Satoh T, Miura M. Top. Organomet. Chem. 2005;14:1–20. Jun C-H, Park JW. Top. Organomet. Chem. 2007;24:117–143. Necas D, Kotora M. Curr. Org. Chem. 2007;11:1566–1592. Crabtree RH. Chem. Rev. 1985;85:245–269. Kondo T, Mitsudo TA. Chem. Lett. 2005;34:1462–1467. Ruhland K. Eur. J. Org. Chem. 2012:2683–2706. Korotvicka A, Necas D, Kotora M. Curr. Org. Chem. 2012;16:1170–1214. Seiser T, Saget T, Tran DN, Cramer N. Angew. Chem. Int. Ed. 2011;50:7740–7752. Murakami M, Matsuda T. Chem. Commun. 2011;47:1100–1105. Dermenci A, Coe PW, Dong G. Org. Chem. Front. 2014;1:567–581. Xu T, Dermenci A, Dong G. Top. Curr. Chem. 2014 DOI: 10.1007/128_2014_545.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

