Model-assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion
- PMID: 25139005
- PMCID: PMC4371827
- DOI: 10.1105/tpc.114.127761
Model-assisted analysis of sugar metabolism throughout tomato fruit development reveals enzyme and carrier properties in relation to vacuole expansion
Abstract
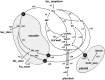
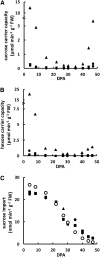
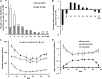
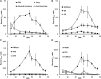
A kinetic model combining enzyme activity measurements and subcellular compartmentation was parameterized to fit the sucrose, hexose, and glucose-6-P contents of pericarp throughout tomato (Solanum lycopersicum) fruit development. The model was further validated using independent data obtained from domesticated and wild tomato species and on transgenic lines. A hierarchical clustering analysis of the calculated fluxes and enzyme capacities together revealed stage-dependent features. Cell division was characterized by a high sucrolytic activity of the vacuole, whereas sucrose cleavage during expansion was sustained by both sucrose synthase and neutral invertase, associated with minimal futile cycling. Most importantly, a tight correlation between flux rate and enzyme capacity was found for fructokinase and PPi-dependent phosphofructokinase during cell division and for sucrose synthase, UDP-glucopyrophosphorylase, and phosphoglucomutase during expansion, thus suggesting an adaptation of enzyme abundance to metabolic needs. In contrast, for most enzymes, flux rates varied irrespectively of enzyme capacities, and most enzymes functioned at <5% of their maximal catalytic capacity. One of the major findings with the model was the high accumulation of soluble sugars within the vacuole together with organic acids, thus enabling the osmotic-driven vacuole expansion that was found during cell division.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures






Comment in
-
Modeling sugar metabolism in tomato fruit.Plant Cell. 2014 Aug;26(8):3222-3. doi: 10.1105/tpc.114.131177. Epub 2014 Aug 26. Plant Cell. 2014. PMID: 25159990 Free PMC article. No abstract available.
References
-
- Amemiya T., Kanayama Y., Yamaki S., Yamada K., Shiratake K. (2006). Fruit-specific V-ATPase suppression in antisense-transgenic tomato reduces fruit growth and seed formation. Planta 223: 1272–1280. - PubMed
-
- Anguenot R., Nguyen-Quoc B., Yelle S., Michaud D. (2006). Protein phosphorylation and membrane association of sucrose synthase in developing tomato fruit. Plant Physiol. Biochem. 44: 294–300. - PubMed
-
- Baker S.M., Schallau K., Junker B.H. (2010). Comparison of different algorithms for simultaneous estimation of multiple parameters in kinetic metabolic models. J. Integr. Bioinform. 7: 133–142. - PubMed
-
- Baxter C.J., Carrari F., Bauke A., Overy S., Hill S.A., Quick P.W., Fernie A.R., Sweetlove L.J. (2005). Fruit carbohydrate metabolism in an introgression line of tomato with increased fruit soluble solids. Plant Cell Physiol. 46: 425–437. - PubMed

