Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome
- PMID: 25139562
- PMCID: PMC4324013
- DOI: 10.1016/j.bbalip.2014.08.006
Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome
Abstract
Acute inflammatory responses are protective, yet without timely resolution can lead to chronic inflammation and organ fibrosis. A systems approach to investigate self-limited (self-resolving) inflammatory exudates in mice and structural elucidation uncovered novel resolution phase mediators in vivo that stimulate endogenous resolution mechanisms in inflammation. Resolving inflammatory exudates and human leukocytes utilize DHA and other n-3 EFA to produce three structurally distinct families of potent di- and trihydroxy-containing products, with several stereospecific potent mediators in each family. Given their potent and stereoselective picogram actions, specific members of these new families of mediators from the DHA metabolome were named D-series resolvins (Resolvin D1 to Resolvin D6), protectins (including protectin D1-neuroprotectin D1), and maresins (MaR1 and MaR2). In this review, we focus on a) biosynthesis of protectins and maresins as anti-inflammatory-pro-resolving mediators; b) their complete stereochemical assignments and actions in vivo in disease models. Each pathway involves the biosynthesis of epoxide-containing intermediates produced from hydroperoxy-containing precursors from human leukocytes and within exudates. Also, aspirin triggers an endogenous DHA metabolome that biosynthesizes potent products in inflammatory exudates and human leukocytes, namely aspirin-triggered Neuroprotectin D1/Protectin D1 [AT-(NPD1/PD1)]. Identification and structural elucidation of these new families of bioactive mediators in resolution has opened the possibility of diverse patho-physiologic actions in several processes including infection, inflammatory pain, tissue regeneration, neuroprotection-neurodegenerative disorders, wound healing, and others. This article is part of a Special Issue entitled "Oxygenated metabolism of PUFA: analysis and biological relevance".
Keywords: Eicosanoid; LC–MS–MS-based targeted lipid mediator metabolomics; Leukocyte; Lipid mediator; Resolvin.
Copyright © 2014 Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures

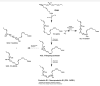



References
-
- Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. - PubMed
-
- Crawford MA, Broadhurst CL, Guest M, Nagar A, Wang Y, Ghebremeskel K, Schmidt WF. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins Leukot Essent Fatty Acids. 2013;88:5–13. - PubMed
-
- Lands WEM. Fish,Omega-3 and Human Health. 2nd. AOCS Press; Champaign, IL: 2005.
-
- Salem N, Jr, Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

