Cerebellar control of gait and interlimb coordination
- PMID: 25139623
- PMCID: PMC4575700
- DOI: 10.1007/s00429-014-0870-1
Cerebellar control of gait and interlimb coordination
Abstract
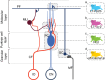
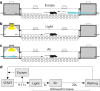
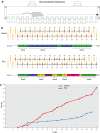
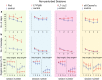
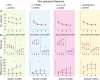
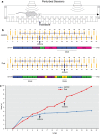
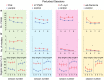
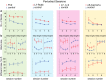
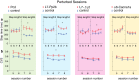
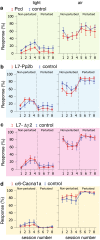
Synaptic and intrinsic processing in Purkinje cells, interneurons and granule cells of the cerebellar cortex have been shown to underlie various relatively simple, single-joint, reflex types of motor learning, including eyeblink conditioning and adaptation of the vestibulo-ocular reflex. However, to what extent these processes contribute to more complex, multi-joint motor behaviors, such as locomotion performance and adaptation during obstacle crossing, is not well understood. Here, we investigated these functions using the Erasmus Ladder in cell-specific mouse mutant lines that suffer from impaired Purkinje cell output (Pcd), Purkinje cell potentiation (L7-Pp2b), molecular layer interneuron output (L7-Δγ2), and granule cell output (α6-Cacna1a). We found that locomotion performance was severely impaired with small steps and long step times in Pcd and L7-Pp2b mice, whereas it was mildly altered in L7-Δγ2 and not significantly affected in α6-Cacna1a mice. Locomotion adaptation triggered by pairing obstacle appearances with preceding tones at fixed time intervals was impaired in all four mouse lines, in that they all showed inaccurate and inconsistent adaptive walking patterns. Furthermore, all mutants exhibited altered front-hind and left-right interlimb coordination during both performance and adaptation, and inconsistent walking stepping patterns while crossing obstacles. Instead, motivation and avoidance behavior were not compromised in any of the mutants during the Erasmus Ladder task. Our findings indicate that cell type-specific abnormalities in cerebellar microcircuitry can translate into pronounced impairments in locomotion performance and adaptation as well as interlimb coordination, highlighting the general role of the cerebellar cortex in spatiotemporal control of complex multi-joint movements.
Keywords: Erasmus Ladder; Granule cells; Interlimb coordination; Interneurons; Locomotion; Purkinje cells.
Figures




Similar articles
-
Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning.Neuron. 2010 Aug 26;67(4):618-28. doi: 10.1016/j.neuron.2010.07.009. Neuron. 2010. PMID: 20797538 Free PMC article.
-
Expression of protein kinase C inhibitor blocks cerebellar long-term depression without affecting Purkinje cell excitability in alert mice.J Neurosci. 2001 Aug 1;21(15):5813-23. doi: 10.1523/JNEUROSCI.21-15-05813.2001. J Neurosci. 2001. PMID: 11466453 Free PMC article.
-
Long-term potentiation of inhibitory synaptic transmission onto cerebellar Purkinje neurons contributes to adaptation of vestibulo-ocular reflex.J Neurosci. 2013 Oct 23;33(43):17209-20. doi: 10.1523/JNEUROSCI.0793-13.2013. J Neurosci. 2013. PMID: 24155325 Free PMC article.
-
[Mechanisms of locomotor control in the cerebellum].Brain Nerve. 2010 Nov;62(11):1149-56. Brain Nerve. 2010. PMID: 21068451 Review. Japanese.
-
Role of the cerebellum and motor cortex in the regulation of visually controlled locomotion.Can J Physiol Pharmacol. 1996 Apr;74(4):443-55. Can J Physiol Pharmacol. 1996. PMID: 8828890 Review.
Cited by
-
Differential deficits in spatial and temporal interlimb coordination during walking in persons with incomplete spinal cord injury.Gait Posture. 2020 Jan;75:121-128. doi: 10.1016/j.gaitpost.2019.10.023. Epub 2019 Oct 17. Gait Posture. 2020. PMID: 31675554 Free PMC article.
-
Somatosensory Cortex Plays an Essential Role in Forelimb Motor Adaptation in Mice.Neuron. 2017 Mar 22;93(6):1493-1503.e6. doi: 10.1016/j.neuron.2017.02.049. Neuron. 2017. PMID: 28334611 Free PMC article.
-
Inducible expression of human C9ORF72 36x G4C2 hexanucleotide repeats is sufficient to cause RAN translation and rapid muscular atrophy in mice.Dis Model Mech. 2021 Jan 11;14(2):dmm044842. doi: 10.1242/dmm.044842. Online ahead of print. Dis Model Mech. 2021. PMID: 33431483 Free PMC article.
-
Locomotor mal-performance and gait adaptability deficits in sickle cell mice are associated with vascular and white matter abnormalities and oxidative stress in cerebellum.Brain Res. 2020 Nov 1;1746:146968. doi: 10.1016/j.brainres.2020.146968. Epub 2020 Jun 10. Brain Res. 2020. PMID: 32533970 Free PMC article.
-
Cerebellar granule cell signaling is indispensable for normal motor performance.Cell Rep. 2023 May 30;42(5):112429. doi: 10.1016/j.celrep.2023.112429. Epub 2023 May 3. Cell Rep. 2023. PMID: 37141091 Free PMC article.
References
-
- Angeby-Moller K, Berge OG, Hamers FP. Using the CatWalk method to assess weight-bearing and pain behaviour in walking rats with ankle joint monoarthritis induced by carrageenan: effects of morphine and rofecoxib. J Neurosci Methods. 2008;174(1):1–9. doi: 10.1016/j.jneumeth.2008.06.017. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

