Assembly and dynamics of the autophagy-initiating Atg1 complex
- PMID: 25139988
- PMCID: PMC4156731
- DOI: 10.1073/pnas.1407214111
Assembly and dynamics of the autophagy-initiating Atg1 complex
Abstract
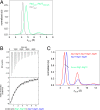
The autophagy-related 1 (Atg1) complex of Saccharomyces cerevisiae has a central role in the initiation of autophagy following starvation and TORC1 inactivation. The complex consists of the protein kinase Atg1, the TORC1 substrate Atg13, and the trimeric Atg17-Atg31-Atg29 scaffolding subcomplex. Autophagy is triggered when Atg1 and Atg13 assemble with the trimeric scaffold. Here we show by hydrogen-deuterium exchange coupled to mass spectrometry that the mutually interacting Atg1 early autophagy targeting/tethering domain and the Atg13 central domain are highly dynamic in isolation but together form a stable complex with ∼ 100-nM affinity. The Atg1-Atg13 complex in turn binds as a unit to the Atg17-Atg31-Atg29 scaffold with ∼ 10-μM affinity via Atg13. The resulting complex consists primarily of a dimer of pentamers in solution. These results lead to a model for autophagy initiation in which Atg1 and Atg13 are tightly associated with one another and assemble transiently into the pentameric Atg1 complex during starvation.
Keywords: analytical ultracentrifugation; intrinsically disordered proteins; isothermal titration calorimetry; membrane tethering; protein structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



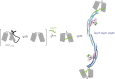
References
-
- Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of autophagosome biogenesis. Curr Biol. 2012;22(1):R29–R34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

