Peritoneal air exposure elicits an intestinal inflammation resulting in postoperative ileus
- PMID: 25140117
- PMCID: PMC4129966
- DOI: 10.1155/2014/924296
Peritoneal air exposure elicits an intestinal inflammation resulting in postoperative ileus
Abstract
Background: The pathogenesis of postoperative ileus (POI) is complex. The present study was designed to investigate the effects of peritoneal air exposure on the POI intestinal inflammation and the underlying mechanism.
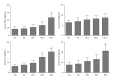
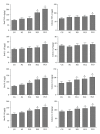
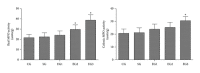
Methods: Sprague-Dawley rats were randomized into five groups (6/group): the control group, the sham group, and three exposure groups with peritoneal air exposure for 1, 2, or 3 h. At 24 h after surgery, we analyzed the gastrointestinal transit, the serum levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and IL-10, the myeloperoxidase activity, and the levels of TNF-α, IL-1β, IL-6, and IL-10 in the ileum and colon. The oxidant and antioxidant levels in the ileum and colon were analyzed by measuring malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total antioxidant capacity (T-AOC).
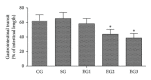
Results: Peritoneal air exposure caused an air-exposure-time-dependent decrease in the gastrointestinal transit. The length of peritoneal air exposure is correlated with the severity of both systemic and intestinal inflammations and the increases in the levels of MDA, SOD, GSH-Px, and T-AOC.
Conclusions: The length of peritoneal air exposure is proportional to the degree of intestinal paralysis and the severity of intestinal inflammation, which is linked to the oxidative stress response.
Figures

References
-
- Rychter J, Clave P. Intestinal inflammation in postoperative ileus: pathogenesis and therapeutic targets. Gut. 2013;62(11):1534–1535. - PubMed
-
- Vather R, Trivedi S, Bissett I. Defining postoperative ileus: results of a systematic review and global survey. Journal of Gastrointestinal Surgery. 2013;17(5):962–972. - PubMed
-
- Prasad M, Matthews JB. Deflating postoperative ileus. Gastroenterology. 1999;117(2):489–492. - PubMed
-
- Asgeirsson T, El-Badawi KI, Mahmood A, Barletta J, Luchtefeld M, Senagore AJ. Postoperative ileus: it costs more than you expect. Journal of the American College of Surgeons. 2010;210(2):228–231. - PubMed
-
- Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. 2009;58(9):1300–1311. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

