δ-Tocotrienol oxazine derivative antagonizes mammary tumor cell compensatory response to CoCl2-induced hypoxia
- PMID: 25140303
- PMCID: PMC4129965
- DOI: 10.1155/2014/285752
δ-Tocotrienol oxazine derivative antagonizes mammary tumor cell compensatory response to CoCl2-induced hypoxia
Abstract
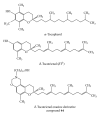
In response to low oxygen supply, cancer cells elevate production of HIF-1α, a hypoxia-inducible transcription factor that subsequently acts to stimulate blood vessel formation and promote survival. Studies were conducted to determine the role of δ-tocotrienol and a semisynthetic δ-tocotrienol oxazine derivative, compound 44, on +SA mammary tumor cell hypoxic response. Treatment with 150 µM CoCl2 induced a hypoxic response in +SA mammary tumor cells as evidenced by a large increase in HIF-1α levels, and combined treatment with compound 44 attenuated this response. CoCl2-induced hypoxia was also associated with a large increase in Akt/mTOR signaling, activation of downstream targets p70S6K and eIF-4E1, and a significant increase in VEGF production, and combined treatment with compound 44 blocked this response. Additional in vivo studies showed that intralesional treatment with compound 44 in BALB/c mice bearing +SA mammary tumors significantly decreased the levels of HIF-1α, and this effect was associated with a corresponding decrease in Akt/mTOR signaling and activation of downstream targets p70S6 kinase and eIF-4E1. These findings demonstrate that treatment with the δ-tocotrienol oxazine derivative, compound 44, significantly attenuates +SA mammary tumor cell compensatory responses to hypoxia and suggests that this compound may provide benefit in the treatment of rapidly growing solid breast tumors.
Figures







References
-
- Holmgren L. Antiangiogenesis restricted tumor dormancy. Cancer and Metastasis Reviews. 1996;15(2):241–245. - PubMed
-
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Molecular Pharmacology. 2006;70(5):1469–1480. - PubMed
-
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annual Review of Cell and Developmental Biology. 1999;15:551–578. - PubMed
-
- Semenza GL. HIF-1: using two hands to flip the angiogenic switch. Cancer and Metastasis Reviews. 2000;19(1-2):59–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

