Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers
- PMID: 25141178
- PMCID: PMC4209186
- DOI: 10.1038/nature13633
Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers
Abstract
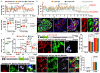
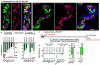
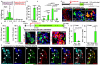
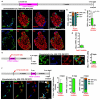
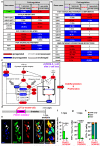
Total or near-total loss of insulin-producing β-cells occurs in type 1 diabetes. Restoration of insulin production in type 1 diabetes is thus a major medical challenge. We previously observed in mice in which β-cells are completely ablated that the pancreas reconstitutes new insulin-producing cells in the absence of autoimmunity. The process involves the contribution of islet non-β-cells; specifically, glucagon-producing α-cells begin producing insulin by a process of reprogramming (transdifferentiation) without proliferation. Here we show the influence of age on β-cell reconstitution from heterologous islet cells after near-total β-cell loss in mice. We found that senescence does not alter α-cell plasticity: α-cells can reprogram to produce insulin from puberty through to adulthood, and also in aged individuals, even a long time after β-cell loss. In contrast, before puberty there is no detectable α-cell conversion, although β-cell reconstitution after injury is more efficient, always leading to diabetes recovery. This process occurs through a newly discovered mechanism: the spontaneous en masse reprogramming of somatostatin-producing δ-cells. The juveniles display 'somatostatin-to-insulin' δ-cell conversion, involving dedifferentiation, proliferation and re-expression of islet developmental regulators. This juvenile adaptability relies, at least in part, upon the combined action of FoxO1 and downstream effectors. Restoration of insulin producing-cells from non-β-cell origins is thus enabled throughout life via δ- or α-cell spontaneous reprogramming. A landscape with multiple intra-islet cell interconversion events is emerging, offering new perspectives for therapy.
Figures








Comment in
-
Diabetes: reprogrammed pancreatic δ-cells restore insulin production.Nat Rev Endocrinol. 2014 Nov;10(11):638. doi: 10.1038/nrendo.2014.158. Epub 2014 Sep 2. Nat Rev Endocrinol. 2014. PMID: 25178729 No abstract available.
References
-
- Al-Masri M, et al. Effect of forkhead box O1 (FOXO1) on beta cell development in the human fetal pancreas. Diabetologia. 2010;53:699–711. doi:10.1007/s00125-009-1632-0. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

